Species Guidelines on Anesthesia and Analgesia
Foundational Concepts & Definitions
- Anesthesia: complete loss of sensation, including the feeling of pain.
- General anesthesia: induced state of the central nervous system depression to unconsciousness in which there is a loss of sensation throughout the body. General anesthetics are frequently given by inhalation or injection.
- Regional anesthesia: anesthesia produced in a region of the body, such as the flank; consciousness may still be present.
- Local anesthesia: anesthesia of a more localized part of the body, such as a digit or tail; consciousness may still be present.
- Anesthetic depth: the degree to which an anesthetic depresses the central nervous system.
- “Surgical” anesthetic plane: defined as the anesthetic level required to accomplish a surgical (or medical) procedure. Commonly, an animal will have no response to a toe pinch, tail pinch, or palpebral reflex; the corneal reflex should always be present.
- Anesthetics: pharmacologic agents required for surgical and medical procedures to help alleviate pain and distress.
- Inhalation anesthetic: a vapor that, when inhaled, produces a state of general anesthesia. Typically, a volatile agent (e.g., isoflurane) is vaporized to allow it to be inhaled.
- Injectable anesthetic: an infusion method to introduce fluid into the body using a needle and syringe. Most common routes of injection include intravenous (IV), subcutaneous (SC), intraperitoneal (IP), and intramuscular (IM).
- Routes of injectable administration:
- Intravenous (IV): administration directly into the venous circulation via a needle or intravenous catheter.
- Intraperitoneal (IP): administration into the abdominal cavity, but not into abdominal organs.
- Subcutaneous (SC): administration between the skin and underlying muscles, where it is absorbed into the vasculature surrounding the injection site. Absorption and distribution within the body is slower than for IV, IM, and IP routes.
- Intramuscular (IM): administration into a large muscle mass where it is absorbed into the vasculature surrounding the injection site.
- Routes of injectable administration:
Pre-Anesthetic, Analgesic, and General Anesthetic Agents
- When choosing a sedative, analgesic, or anesthetic, it is important to consider the following:
- Ease of administration
- How it is metabolized
- The level of distress it causes the animal
- The degree of analgesia it provides
- Associated quality and rate of recovery
- Types of agents
- Injectable (Pre-Anesthetic, Analgesic, Induction Agents)
- Inhalant
- It is very important to consult your RARC veterinarian when determining what anesthetic is best for your study.
Injectable Agents
Pre-Anesthetics
- Reasons to use pre-anesthetics:
- Decrease stress
- Provide sedation, restraint, and analgesia
- Minimize autonomic reflex activity
- Decrease other drug (including inhalant) requirements
Examples (for detailed information, see Lamont et al, The Sixth Edition of Lumb & Jones):
Alfaxalone (also used as an anesthetic induction agent, depending on dose)
-
- Neuroactive steroid
- Routes of administration: IM or IV (pH = 7.0)
- Enhances GABA receptor-mediated inhibitory neurotransmission
- Rapidly metabolized with minimal accumulation
- Physiologic effects:
- Dose-dependent cardiovascular depression but blood pressure is better maintained due to reflex increases in heart rate
- Dose-dependent respiratory depression; apnea is common
- Muscle twitching at low or inadequate doses when given via IM injection
- Rough recoveries in some species
Alpha-2 adrenoreceptor agonists (xylazine, dexmedetomidine, detomidine)
-
- Inhibits norepinephrine release in the CNS
- Profound sedation
- Mild analgesic properties (visceral)
- Physiologic effects:
- Muscle relaxation
- Profound CV depression (bradycardia, decreased cardiac output, increased vasoconstriction)
- Hypertension followed by hypotension
- Potential respiratory depression and pulmonary edema in ruminants
- Hyperglycemia, diuresis
- Reversal agent (antagonist) available: atipamezole
Anticholinergics/parasympatholytics (atropine, glycopyrrolate)
-
- Compete with ACh at muscarinic receptors
- Reduce vagal responses – increase heart rate
- Increase viscosity of secretions
- Decrease GI motility
- Cause tachycardia (may increase cardiac work)
- Not routinely used as pre-anesthetic agents
Benzodiazepines (midazolam, diazepam, zolazepam [part of Telazol®])
-
- Augments GABA binding (main inhibitory CNS neurotransmitter)
- WIDE species differences, from sedation to excitement
- Anticonvulsant
- Physiologic effects:
- Muscle relaxation
- Minimal cardiovascular and respiratory depression at clinical doses
- Reversal agent available: flumazenil
- Diazepam is only administered IV; propylene glycol in the formulation results in erratic absorption when given via IM, IP, or SC routes
Dissociatives (also used as anesthetic induction agents, depending on dose: ketamine, tiletamine [part of Telazol®])
-
- NMDA receptor antagonists
- Tiletamine – slightly more potent; longer duration than ketamine
- IM/SC/IP administration is painful due to pH (3.5-5.5)
- Moderate analgesia (even in low doses or constant rate infusions)
- Physiologic effects:
- Sympathetic effects: maintain cardiac output, blood pressure, reflexes (laryngeal, corneal)
- Use caution in heart disease especially in conjunction with alpha-2 agonists due to potential tachycardia with vasoconstriction increasing myocardial work and oxygen consumption
- Minimal respiratory depression but apneustic breathing seen (breath held in inspiration)
- Poor muscle relaxation – add midazolam, alpha-2 agonist, etc. which have muscle relaxant properties
Phenothiazines (acepromazine)
-
- Dopaminergic and alpha-adrenergic antagonists
- Slow onset and long duration of action
- Anti-emetic (may reduce vomiting), anti-arrhythmic (helps to reduce epinephrine-induced irregular heart rhythms), anti-histaminic (may reduce allergic reactions)
- Physiologic effects:
- Vasodilation resulting in potential low blood pressure (hypotension)
- Hypothermia
- Mild sedation
- No analgesic properties and no reversal agents available
Analgesic Agents
Opioids
- Analgesia mainly via mu- and kappa-opioid receptors
- Reversal agents available: naloxone (mu- and kappa-opioid receptor antagonist), butorphanol (mu-opioid receptor antagonist)
- Physiologic effects:
- Bradycardia (otherwise, minimal cardiovascular effects)
- Respiratory depression
- Urinary retention
- Vomiting (in some species)
- Ileus
- Pica (rodents)
- Hyperthermia (in some species)
- Potential hyperalgesia
- Specific opioids
- Buprenorphine: partial mu-opioid receptor agonist, 4-12 hr duration, moderate analgesia
- Morphine, hydromorphone: full mu-opioid receptor agonists, 2-4 hr duration, profound analgesia
- Fentanyl: full mu-opioid receptor agonist, < 1 hr duration after IV bolus, profound analgesia; best administered as a patch or constant rate infusion (CRI)
- Butorphanol: mu-opioid receptor antagonist, kappa-opioid receptor agonist, 1-2 hr duration, mild analgesia
- Tramadol: weak mu-opioid receptor agonist, NE and 5-HT reuptake inhibitor, weak analgesic with minimal clinical benefit when delivered orally in some species (dogs), used only as an adjunct in others (rodents)
Local anesthetics
- Block sodium channels in autonomic (sympathetic), afferent (sensory), and efferent (motor) nerves
- Delivered locally, regionally, topically, epidurally, etc. depending on specific procedure
- Exhibit analgesic/anti-hyperalgesic properties and block "wind-up"
- Wind-up pain results from neuroplastic changes associated with repeated stimulation of pain pathways. It leads to an increased excitability of neurons, resulting in heightened pain responses to normally painful stimuli or even non-painful stimuli. Wind-up pain can be present even when the painful stimulus is completely removed.
- Toxicity can cause sedation, nausea, vomiting, cardiotoxicity, seizures, death
- Specific local anesthetics:
- Lidocaine: short onset and duration (1-2 hr)
- Bupivacaine: longer onset and duration (4-6 hr)
- Ropivacaine: longer onset and duration (4-6 hr)
- Liposomal encapsulated bupivacaine (Nocita®): up to 3 days of analgesia in certain circumstances
Non-Steroidal Anti-inflammatory Drugs (NSAIDs)
- Inhibit COX enzymes associated with inflammation (induced and constitutively expressed)
- Examples:
- Non-specific COX-1 and COX-2 inhibitors: aspirin, acetaminophen, flunixin, ibuprofen, ketoprofen, naproxen, phenylbutazone
- Selective/specific COX-2 inhibitors: carprofen, deracoxib, meloxicam, firocoxib, robenacoxib
- Useful in acute, chronic, and post-op inflammatory pain
- Associated with GI, renal, hematologic, and hepatic side effects
- Use with extreme caution (or not at all) in patients with concurrent corticosteroids, decreased circulatory blood volume, shock, dehydration, hypotension, and GI disease
Gabapentin
- Does not bind GABA receptors – acts at alpha-2 delta subunit of pre-synaptic Ca++ channels to decrease excitatory neurotransmitter release
- Use in conjunction with NSAIDS, opioids, etc. to improve their analgesia
- Side effects include sedation in some species and ataxia
- Minimal post-surgical analgesia, but useful to reduce hyperalgesic states
Others
- Other drugs used as pre-anesthetic or anesthetic induction agents possess analgesic properties including ketamine, dexmedetomidine, etc. Please contact your RARC veterinarian concerning the use of these drugs for analgesia in your protocols.
Anesthetic Induction Agents
Alfaxalone (see Pre-Anesthetics above)
- Neuroactive steroid
- May decrease blood pressure due to vasodilation, but reflex increases in heart rate may maintain blood pressure
Ketamine/tiletamine Alfaxalone (see Pre-Anesthetics above)
- NMDA receptor antagonists; also called "dissociative anesthetics"
- May cause apneustic breathing
- May maintain heart rate and blood pressure, but also may increase myocardial work and oxygen consumption
Propofol (non-barbiturate, non-steroid)
- Enhances GABA receptor-mediated inhibitory neurotransmission
- Administered IV only (formulated in egg lecithin and soybean oil)
- Respiratory and cardiovascular depression
- Minimally cumulative due to hepatic and extra-hepatic metabolism
- Propofol 28 has a longer shelf life (benzyl alcohol preservative included)
Tricaine methanesulphonate (MS-222)
- Dissolved in water; mainly used in fish and amphibians
Urethane
- Long acting
- Carcinogenic
- Good cardiovascular, neurologic, and respiratory stability
- Used primarily for non-survival procedures
Not currently recommended: α-chloralose, chloral hydrate, tribromoethanol (Avertin)
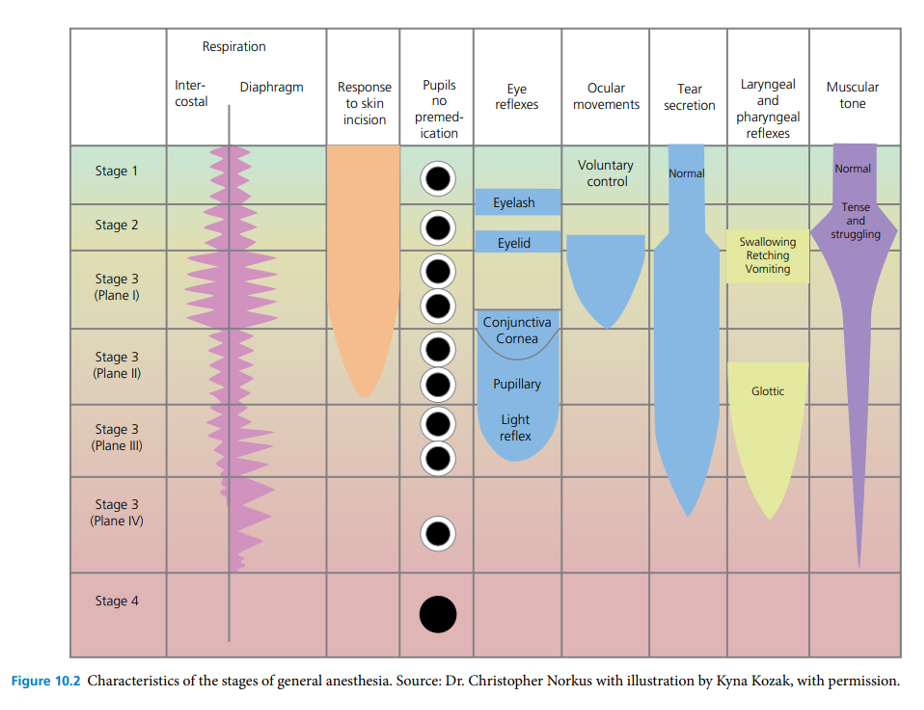
Stages of Inhalant Anesthesia
Animals progress through different stages of anesthesia as they become anesthetized at the beginning and as they recover from the anesthetic procedure. Changes in breathing, eye and upper airway (laryngeal) reflexes, response to skin sensation, and voluntary movement/motor tone occur as each plane changes; these are discussed below.
Stage I: The stage of voluntary movement
- This stage starts from initial anesthetic administration and ends with loss of consciousness. Some animals may struggle and become stressed in this initial stage. During this stage, epinephrine release causes an increase in heart rate and the pupils may dilate. Urination, defecation, and salvation increase in some species. As stage II is approached, animals lose their ability to stand and the righting reflex (ability to right themselves off their side from lateral recumbency).
Stage II: The stage of involuntary movement/delirium
- During this stage, commonly referred to as the “excitement stage”, the central nervous system (CNS) response progressively becomes downregulated. This stage covers the time between unconsciousness and the start of a regular breathing pattern. Animals will react strongly to external stimuli and may hyperventilate with abnormally frequent shallow breaths (tachypnea) or may hold their breath. Continued catecholamine release may cause a rapid heart rate, irregular cardiac rhythm, and extreme pupillary dilation. During this stage, palpebral reflexes are commonly observed, and animals may vocalize depending on the species. Excessive salivation and vomiting may occur in some species. The goal is to quickly pass through this stage.
Stage III: The stage of surgical anesthesia
- Animals at this stage are unconscious and reflexes are gradually lost. Muscle tone decreases and breathing becomes slower and more regular. Animals lose the ability to voluntarily swallow and vomit.
- Depth of surgical anesthesia has been classified into three subcategories: light (Plane 1), medium (Plane 2), and deep (Planes 3 and 4).
- Plane 1: Light anesthetic depth lasts until eye movement stops. Withdrawal reflexes may still be present.
- Plane 2: Medium anesthetic depth is considered adequate for surgery. Features of medium depth include a regular breathing pattern and pulse rate, slow or absent palpebral reflex with a strong corneal reflex, absent laryngeal reflexes, adequate muscle relaxation, and acceptable analgesia. This is the acceptable plane during surgery, although cardiac output and ventilation may become depressed.
- Planes 3 and 4: Deep surgical anesthesia is characterized by progressive profound respiratory depression with decreased tidal volume and breathing rate, profound cardiovascular depression with low blood pressure, strong muscle relaxation, weak corneal reflex, and dilated pupils.
Stage IV: Extreme CNS Depression and Overdose
- This plane requires emergency intervention as cardiac and pulmonary function fails. Respiration stops and the heart continues to beat for only a short period. Blood pressure is extremely low and capillary refill of mucous membranes may be significantly delayed. Pupils remain greatly dilated and reflexes and muscle tone are absent. This stage results in death unless intervention is taken in the form of resuscitation.
Characteristics of the Stages of General Anesthesia
Veterinary Anesthesia and Analgesia (Lamont et al.)

Inhalant Anesthetics
Considerations:
- Inhalants may be used for anesthetic induction (via chamber or mask/nosecone) or maintenance (via endotracheal tube or mask/nosecone).
- Compared to injectable anesthetics, inhalant agents result in more rapid anesthetic depth adjustments and generally a more stable anesthetic plane.
- Inhalants are eliminated from the body primarily by exhalation and rely less on metabolism.
- Pre-anesthetic agents are recommended prior to inhalant agent induction or maintenance.
- Current agents are nonflammable liquids that provide good muscle relaxation but no analgesia (isoflurane, sevoflurane); pain pathways are still activated during inhalant anesthesia and analgesics must be administered in painful procedures.
Specific agents (for detailed information, see Lamont et al, The Sixth Edition of Lumb & Jones):
Isoflurane
- Has low blood:gas solubility for fast induction/recovery.
- Most is eliminated through the lungs with a small amount metabolized by the liver (~0.2-0.3%).
- Dose-dependent decreases in arterial blood pressure due to significant vasodilation and reduced cardiac contractility.
- Dose-dependent respiratory depression with decreased tidal volume and increased arterial (and end-tidal) CO2 concentrations.
- Increases cerebral blood flow and intracranial pressure.
Sevoflurane
- Physiologic effects are similar to isoflurane with significant cardiovascular and respiratory depression.
- Although primarily exhaled from the body, ~2-3% is metabolized by the liver to inorganic fluoride ions; however, clinical kidney and hepatic toxicities are not noted with normal anesthetic delivery.
- Compound A, a nephrotoxin, may be produced when it contacts specific carbon dioxide absorbents.
- May have slightly quicker induction and recovery than isoflurane due to very low blood:gas solubility (clinical differences may be minimal).
- Separate vaporizers are required for isoflurane and sevoflurane due to their unique vapor pressures.
Nitrous oxide
- Inhaled agent with minimal cardiovascular and respiratory depressant effects and mild analgesic properties.
- Weak anesthetic agent in research animal species (very high minimal alveolar concentration [MAC] values) which differs from its use in humans.
- Quickly diffuses into gas-filled spaces such as intestines; not absorbed by charcoal filters; chronic use is associated with myelin and blood disorders.
- Used as an adjunctive anesthetic and carrier gas in combination with oxygen to avoid hypoxemia, not as a sole agent.
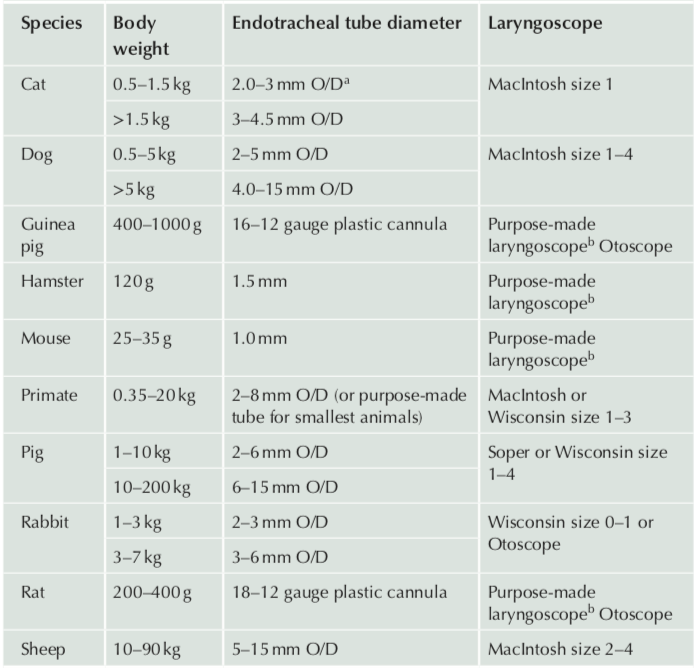
Endotracheal Intubation
- Endotracheal tubes are used to prevent physiologic upper airway obstruction, facilitate delivery of anesthetic, and transport gases to and from the lungs. They can also be used to assist breathing if needed.
- Laryngeal/swallowing reflexes are abolished at surgical planes of anesthesia. Therefore, cuffed endotracheal tubes may provide at least partial protection for the airway from gastrointestinal contents when cuffs are not under- or over-inflated. There are many different sizes based on the size and species of your animal. However, many small tubes, including rodent tubes, are uncuffed.
- Laryngoscopes (or other visual aids such as fiberoptic scopes) are used to ensure appropriate visualization of the larynx so that endotracheal tube passage into the trachea is minimally traumatic and that the tube does not enter the esophagus, potentially stimulating unwanted reflux or regurgitation. Laryngoscope handles and blades come in many sizes and varieties based on your application and the species you are working with.
- Endotracheal tubes, when placed properly, should glide down the larynx/trachea easily; force should be avoided when passing the tube to reduce tissue damage. If the tube does not pass easily, readjust the angle of the tube. The largest tube possible should be used.
Suggested Equipment for Endotracheal Intubation by Species (Flecknell 2016)
Anesthetic Monitoring
Three elements of successful monitoring:
- Monitor for normal physiological responses from the beginning to easily recognize any abnormal discrepancies that may occur throughout the anesthetic process.
- Correctly interpret any changes to the normal physiological responses. By having detailed knowledge of your species and the specific surgical/anesthetic event, it is easier to recognize abnormal physiological responses.
- Provide proper means of intervention to the patient in the event of an abnormal physiological response. Examples of intervention include drug and/or supportive therapy and adjustment in anesthetic levels.
Anesthetic monitoring techniques:
- Although constant vigilance is required during anesthesia, recording vital signs every 5-10 minutes is recommended, and in some species, required to immediately see values and to assess changes over time.
- Vital signs
- Signs that should be monitored for large animal anesthesia include heart rate and rhythm, capillary refill time (CRT), mucous membrane color, blood pressure, end-tidal CO2 (ETCO2), SPO2 (pulse oximetry), electrocardiography, blood loss, respiratory rate, pulse pressure and temperature. However, specific monitoring techniques appropriate for your model should be discussed with veterinary personnel.
- For smaller laboratory animals (e.g., rodents), required vital sign monitoring may be adapted from these.
- Cardiovascular system:
- Heart rate
- Impacts the cardiac output, which is the product of heart rate and stroke volume.
- Bradycardia (slower heart rate): potentially caused by specific anesthetic drugs, hypothermia, cardiovascular collapse, or hypertension.
- Tachycardia (faster heart rate): potentially caused by specific drugs (atropine, glycopyrrolate, epinephrine, ketamine, etc.), hypotension, hypoxia, hypovolemia, hyperthermia, or hypercarbia (high CO2 levels). It could also be caused by a painful stimulus during surgery in a lightly anesthetized animal.
- Pulse quality and rate
- Can be taken from several sites (tail artery, auricular artery, lingual artery, femoral artery). The site is species-dependent.
- Palpation of a pulse can provide you information regarding heart rhythms.
- Arterial blood pressure
- Estimates organ perfusion and cardiovascular function.
- Indirect/non-invasive (Doppler or automatic oscillometric) readings or direct/invasive (arterial catheter) methods can be used.
- Normal, conscious values vary by species but approach systolic ~ 120 mmHg, diastolic ~ 80 mmHg, mean ~ 100 mmHg.
- Values are frequently much lower during inhalant anesthesia, but mean blood pressure must remain > 60-70 mmHg for organ perfusion.
- Heart rate
- Respiratory system:
- Respiratory rate
- Measured by visualizing chest wall movement as it rises and falls, by watching the rebreathing bag on the anesthesia machine, or via the ETCO2 monitor (capnometer).
- Normal respiratory rates are species-dependent.
- Tidal volume
- Definition: the volume of air inhaled or exhaled in a single breath during normal respiration.
- Measured by using a respirometer which is attached to an endotracheal tube.
- For most species, the average tidal volume is ~10-15 mL/kg.
- Minute ventilation
- Minute ventilation= respiratory rate x tidal volume
- Major determinant of arterial (and end-tidal) CO2 levels and determines whether an animal is hypo- or hyper-ventilating.
- High minute ventilation results in lower CO2 levels and low minute ventilation results in high CO2 levels.
- Capnometry/capnography
- The amount of inhaled and exhaled carbon dioxide is measured by capnometry whereas capnography graphs the ventilatory cycle waveform, allowing further physiologic assessment.
- ETCO2 is a product of cellular metabolism, circulation to the lung, and minute ventilation.
- The capnograph samples CO2 levels as it is connected to the end of the endotracheal tube (although it varies by species, normal = ~35-45 mmHg).
- Essential to determine adequacy of cardiopulmonary resuscitation (CPR).
- Pulse oximetry
- Measures the amount of hemoglobin saturated with oxygen and the pulse rate/rhythm.
- Veterinary devices specific to your species are recommended.
- Arterial blood gas analysis
- The definitive method of analyzing adequacy of ventilation and oxygenation.
- Respiratory rate
- Anesthetic depth
- Determination of CNS depth is dependent upon:
- Animal species (see the tab above for your particular species)
- Type/invasiveness of surgery or procedure being performed
- Other drugs administered (analgesics, pre-anesthetics, etc.)
- Methods of determining anesthetic depth include reflex assessment (palpebral reflex, toe pinch, muscle tone, etc.) as well as physiologic assessment as discussed above.
- Determination of CNS depth is dependent upon:
- Body temperature
- Many species will quickly decrease core body temperature immediately upon anesthetic induction although hyperthermia may also occur at times.
- Rectal or esophageal thermometers are frequently used (specific probes are used for each species).
- Temperature should be controlled throughout the procedure and into anesthetic recovery by use of reduced oxygen flow rates, circulating warm water blankets, forced warm air circulators, and warmed anesthetic circuits.
- Minimizing excessive use of surgical clipping and scrubbing or prepping agents, along with maintaining warm ambient temperatures, are the most efficacious in preventing heat loss.
- Electrical blankets, hot plates, rice bags, water bottles, and warmed gloves are NOT allowed because they frequently produce burns.
- Pain scoring
- Validated pain scoring systems (or adaptations thereof) should be used at intervals appropriate for your procedure and species (see the tab above for your particular species, and the "Analgesia Standard Treatment Guidelines for Laboratory Rats/Mice" links under "Pain Management" in the menu near the top of the page).
- Analgesics must be administered at the appropriate intervention levels as dictated by your protocol.
Fluid Therapy
- To offset fluid losses during the procedure and subsequently prevent dehydration afterwards, fluid delivery and intake should be monitored.
- Intra-operative anesthetic fluid rate varies between species but should be ~3-10 mL/kg/hr IV using balanced crystalloid solutions (Plasmalyte, Normosol, Lactated Ringer’s, etc.). Fluid rates and types should be adjusted based on individual animal needs. Other routes of administration may be required (SC, IP).
- Voluntary fluid intake should be documented after the procedure. Voluntary water intake is commonly reduced after surgery, leading to increased morbidity.
- Once fully recovered, supplemental fluids consisting of a balanced crystalloid (Plasmalyte, Normosol, Lactated Ringer’s, etc.) can be given SC or IP. Oral glucose and electrolyte-containing gels may also be used.
- In some species, dehydration can be observed by the loss of skin tone and elasticity. If an animal is adequately hydrated, the forehead skin should go back to its original state quickly after the skin is tented. In large animals, mucous membranes will become dry to the touch if the animal is dehydrated, and eye position may be altered.
- Maintenance fluid requirements for most species range from 40 to 80 mL/kg in a 24-hour period.
Recovery
Factors that contribute to a successful recovery:
- Environment
- Recovery area should be warm and quiet.
- Lighting should be dimmer than normal, yet bright enough that proper observation of the animal is still easily achieved.
- For most adult animals, the room temperature during recovery should be around 27 °C-30 °C . For some neonatal species, the room temperatures should register around 35 °C-37 °C. If these room temperatures cannot be achieved, the incorporation of supplemental heat sources should be used as above.
- Constant vigilance in recovery is required and direct observation of clinical appearance and recording of vital signs (as applicable) must be performed every 5-10 minutes until fully recovered (sternal recumbency, normal locomotion, etc.).
- Use of post-anesthesia monitoring sheets documenting recovery procedures is required; templates are available on the RARC website.
- Minimizing human handling during recovery
- Animals’ response to human contact and intervention during recovery is highly dependent on species. Some rodents are highly sensitive to human touch and stimulation during anesthetic recovery, creating an aversive reaction. If acclimation to humans occurs prior to surgery and the recovery and the immediate post-operative care is performed in a calm manner, stress can be minimized.
- Proper hydration and gastrointestinal (GI) function
- Fluids should be administered as discussed above and hydration status assessed throughout the recovery period.
- Some species are fasted before a surgical procedure to reduce the contents within the GI system (see the tab above for your particular species). If the animal is not defecating after surgery, it may be due to pain or lack of fecal material in the GI tract. It can also be a sign of reduced GI motility, known as ileus. This can become a significant problem for select species including rabbits, guinea pigs, ruminants, and swine. If ileus is suspected, RARC veterinary personnel should be contacted.
- When fasting ruminants, it should be noted that ingested material is fermented in their rumen for some time and then proceeds to the other compartments of the GI tract for further digestion. Therefore, fasting may not greatly impact GI emptying rate and it is impossible to fully empty the rumen.
- Some species may not eat well after surgery. Supplemental feeding using nasogastric tubes or oral gavage may be necessary and beneficial.
- Like water intake, food intake should be documented and monitored regularly.
Additional Resources
Consult the RARC Veterinary Staff (vet@rarc.wisc.edu) if you have specific questions about the anesthetic procedures included in your IACUC protocol.
For assistance with anesthesia equipment set-up, please contact the RARC Trainers (trainer@rarc.wisc.edu).
Form templates to record anesthesia monitoring are available on the RARC website.
RARC loans out select anesthesia equipment to the UW-Madison research community for temporary use. Please visit the RARC website to find out what items are available to borrow.
Useful Text Resources:
Veterinary Anesthetic and Monitoring Equipment by Cooley and Johnson
UW-Madison Libraries provides free access to the online version of this textbook.
Veterinary Anesthesia and Analgesia: The Sixth Edition of Lumb and Jones by Lamont et al.
UW-Madison Libraries provides free access to the online version of this textbook.
Exotic Animal Formulary by Carpenter et al.
UW-Madison Libraries provides free access to the online version of this textbook.
Works Cited
“Anesthesia (Guideline).” Anesthesia (Guideline)|Vertebrate Animal Research, 10 May 2023. https://animal.research.uiowa.edu/iacuc-guidelines-anesthesia.
Danneman PJ and Fish RE. Anesthesia and Analgesia in Laboratory Animals (Second Edition). Academic Press, 2008.
Flecknell PA. Laboratory Animal Anaesthesia Ed. 4. Academic Press, 2015.
Lamont L et al. Veterinary Anesthesia and Analgesia: The Sixth Edition of Lumb and Jones. Wiley Blackwell, 2024.
“Supplemental Anesthesia Training Resource.” Received by Rebecca Johnson, Supplemental Anesthesia Training Resource, 7 May 2020.
Anesthetic Emergencies
Appropriateness of the below procedures for your animal model should be discussed with veterinary personnel prior to experimentation since, if an anesthetic emergency occurs, the validity of your data may be affected.
The flowchart below states recommended guidance for select physiological abnormalities:
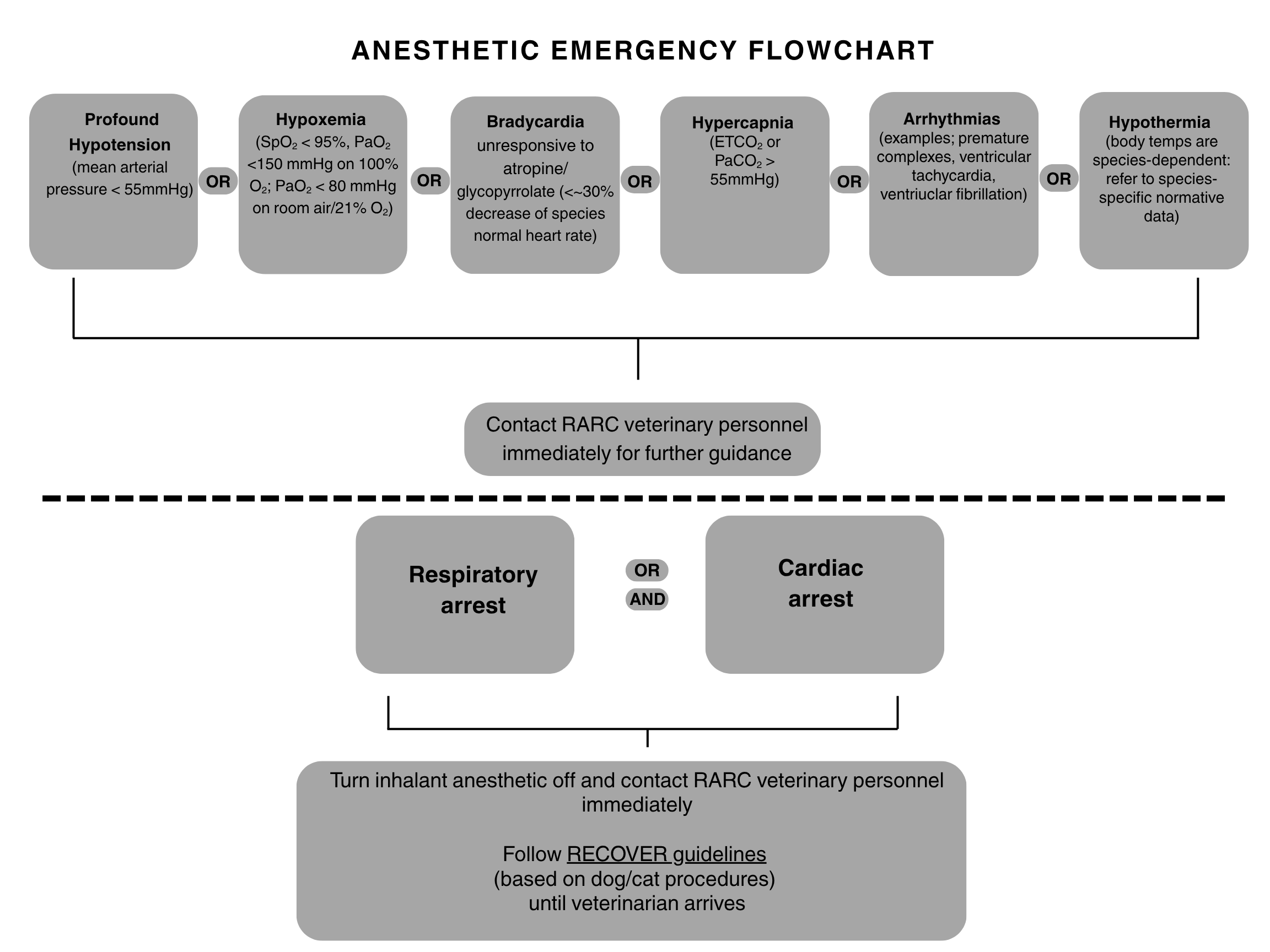
Access the RECOVER guidelines HERE.
Cardiopulmonary arrest is diagnosed when the animal is not breathing and no pulse or heart beat can be heard/palpated. Often, all reflexes such as palpebral and corneal reflexes are lost as well.
Cardiac Support: Begin cardiac compressions at a rate similar to the normal heart rate for the species. For very small animals (rodents, rabbits, etc.), the fingers of one hand may be used across the chest. For larger animals (sheep, swine, etc.) two hands compressing the widest part of the chest should be used.
Airway: Place an endotracheal tube or V-Gel into the trachea if possible. Confirm correct placement by using a capnometer or auscultating breath sounds on both sides of the chest.
Breathing: Ventilate with 100% oxygen or an AMBU bag at a rate similar to the normal respiratory rate of your species. Do NOT overventilate.
For training concerning CPR in your particular species, please contact the RARC Training Team at trainer@rarc.wisc.eduand they will coordinate a session with our veterinary anesthesiologist.
In the event of an emergency, the following agents may be administered as indicated by the situation. Charts with appropriate dosing for your particular species can be provided by RARC staff.
Atropine: Used for vagal-induced bradycardia
Epinephrine: Used in cardiac arrest
Lidocaine: Used if an ECG indicates ventricular arrhythmias
Neuromuscular Blocking Agents (NMBAs)
NMBAs are used to paralyze skeletal muscles during a procedure. ALL skeletal muscles are affected, including the diaphragm. Thus, extreme attention must be taken to ensure that a proper level of anesthesia and analgesia is achieved prior to administering a neuromuscular blocking agent since purposeful movement cannot occur following administration.
Neuromuscular blocking agents require special monitoring procedures; please contact RARC veterinary personnel for guidance regarding monitoring, appropriate agents, and dosing for your particular species and model.
- NMBAs are always administered IV.
- Non-depolarizing NMBAs work by competitive antagonism of acetylcholine (ACh) at the neuromuscular junction.
- Controlled (mechanical) positive pressure ventilation is required.
- End-tidal (or arterial) carbon dioxide level monitoring is required.
- Monitoring neuromuscular transmission through the use of Train-of-Four (TOF) monitoring and accelerometry is recommended.
- Residual neuromuscular blockade with impaired laryngeal function is common. Reversal of NMBAs with neostigmine and glycopyrrolate is possible under specific conditions. Please consult the RARC veterinarians for instructions on NMBA reversal.
Examples of Neuromuscular Blocking Agents
For doses specific to your species, please contact RARC veterinarians.
| Drug | Onset | Duration of Effect | Notes |
| Atracurium | 2-3 minutes | 30-40 minutes, depending on dose | Undergoes Hoffman elimination and does not rely on liver for metabolism Minimal cardiovascular side effects |
| Pancuronium | 2-3 minutes | Multiple hours, depending on dose | Vagolytic (increases heart rate) May result in norepinephrine and histamine release |
| Rocuronium | 2-3 minutes | 5-15 minutes, depending on dose | Mild vagolytic effects Transient hypo- or hypertension |
RARC’s “Overview of Anesthetic and Analgesic Procedures” must be read prior to accessing the below information.
Contact your RARC veterinarian prior to choosing an anesthetic, including agents that are NOT listed below.
Species-Specific Considerations
Pre-Anesthetic Considerations
- An acclimation period of at least 3 days is required prior to any procedure.
- Body weights must be obtained for appropriate drug dosing.
- Birds may become stressed by improper handling and use of volatile anesthetics during the induction phase of anesthesia, leading to respiratory and cardiac arrest.
- Fasting is not required due to the high metabolic rate of birds. However, food may be stored in the crop.
- IM and SC injections are common. The preferred IM injection site is within the pectoral (breast) muscles just lateral to the keel bone.
- Birds do not have a diaphragm; the coelom is the main body cavity that houses all the internal organs, such as the heart, lungs, digestive tract, and reproductive organs.
- The avian respiratory system is highly specialized for efficient gas exchange, particularly for the energy-demanding activity of flight. It has a unique system of air sacs that act as bellows and a unidirectional flow of air through the lungs, unlike the bidirectional flow seen in mammals.
- Birds experience SIGNIFICANT respiratory depression and require controlled ventilation throughout any anesthetic procedure.
- An IV catheter should always be placed in larger birds to administer additional anesthetic agents, emergency drugs and/or fluids. The metatarsal, ulnar or right jugular vein can be used. Intraosseous catheters can also be placed in the distal ulna or proximal tibiotarsus for fluid or drug delivery.
- Despite anesthetic chosen, sterile ophthalmic ointment should be applied to eyes since blink reflexes are significantly reduced during anesthesia and sedation.
- It is very important to consult your RARC veterinarian when determining what anesthetic is best for your study.
- Young birds require unique considerations; please consult your RARC veterinarian for specific recommendations.
Recommended Agents
Injectable Sedatives, Anesthetics, and Analgesics
Sedatives/Anesthetics
- Examples of the most common sedative and anesthetic agents are included below, along with reversal agents. Doses vary based on species; please consult RARC veterinary personnel before choosing agents/doses.
- Detailed information concerning agents can be found at: Lamont et al, The Sixth Edition of Lumb & Jones.
- Premedication is common and is administered IM prior to IV catheterization as discussed above.
- Premedication should be done quickly once the bird is caught up with the bird well-restrained.
- Supplemental oxygen should always be administered throughout sedation and/or anesthesia.
Sedatives Used in Birds
| Drug | Dosage/Route* | Duration | Comments |
| Midazolam (recommended) | 1-4 mg/kg IM, IV | 30-45 minutes | Mild to moderate sedation Dose varies with species Frequently combined with butorphanol Reversed with flumazenil |
| Dexmedetomidine | 0.01-0.06 mg/kg IM | 30-45 minutes | Mild to profound sedation, based on dose Some analgesia Dose varies with species Bradycardia is expected Reversed with atipamezole |
| Ketamine | 10-25 mg/kg IM | 30-40 minutes | Moderate sedation Must combine with other agents (midazolam, dexmedetomidine, etc.) for adequate muscle relaxation Associated with tachycardia |
*Intramuscular (IM), intravenous (IV). These can be used with other agents (such as opioids) to produce analgesia, profound sedation, or general anesthesia as below.
Injectable Anesthetics Used in Birds
| Drug | Dosage/Route* | Anesthetic Duration | Comments |
| Dissociative Combinations | |||
| Ketamine + dexmedetomidine + midazolam |
10-25 mg/kg ketamine + 0.01-0.06 mg/kg dexmedetomidine + 1-6 mg/kg midazolam IM |
Up to 30-45 minutes | Light to moderate anesthetic plane, depending on dose Only used for short, non-invasive procedures Useful prior to euthanasia Midazolam reversed with flumazenil Dexmedetomidine reversed with atipamezole |
| Others | |||
| Propofol | 3-10 mg/kg IV slow bolus 0.1-0.8 mg/kg/min IV constant rate infusion (CRI) |
5-7 minutes | Titrate to effect for anesthetic induction or used as a CRI as part of partial intravenous anesthesia (PIVA) Cardiorespiratory depression; oxygen supplementation and controlled ventilation is recommended following tracheal intubation Apnea is common |
*Intramuscular (IM), intravenous (IV)
Reversal Agents Used in Birds
| Drug | Dosage/Route* | Reversal Category | Reversal for: |
| Atipamezole | 0.5-1 mg/kg IM | Alpha-2 adrenergic receptors | Dexmedetomidine |
| Flumazenil | 0.05-0.1 mg/kg IM, IV | Benzodiazepines | Midazolam |
| Naloxone | 0.1-1 mg/kg IM, IV | Opioids | Butorphanol, etc. |
*Intramuscular (IM), intravenous (IV)
Analgesics
- Examples of common analgesic agents are listed below. Others are available; please contact RARC veterinarians to discuss alternatives.
- Unrelieved pain has profound physiologic consequences, which may alter research results.
- Pain assessment in birds can be challenging. Painful behaviors include decreased food consumption, activity, and preening, weight loss, tucked head, ruffled feathers, closed eyes, crouching, etc. Pain-associated signs should be documented in the record along with any analgesic intervention taken.
- Behavior scoring systems have been developed for certain species and should be used as a guideline for a scoring system unique to your model (Mikoni et al. 2023); scores should be documented in the record along with any analgesic intervention taken.
- Procedures that are presumed to be painful warrant the provision of analgesia, despite the lack of indicators of pain.
- Analgesic agent efficacy, duration and bioavailability vary widely between species (Guzman et al. 2023). For example, earlier studies recommended that kappa-opioid receptor agonists were the opioid class of choice in birds; however, recent studies show that in some avian species full mu-opioid receptor agonists may be more efficacious. Contact RARC veterinary personnel when choosing appropriate analgesic agents for your particular species.
- The IACUC requires the use of preemptive analgesia (analgesics given prior to the first skin incision) for all survival surgical procedures unless scientifically justified.
- Pre-emptive analgesics may decrease the amount of required anesthetic drugs; doses of both injectable and inhalant agents should be adjusted accordingly
Analgesic Agents Used in Birds
| Drug | Dosage/Route* | Frequency | Comments |
| Opioids | |||
| Butorphanol | 1-5 mg/kg IM, IV | 0.5-3 hours | Kappa opioid agonist Mu opioid antagonist |
| Hydromorphone | 0.1-0.6 mg/kg IM | 3-6 hours | Full mu opioid agonist |
| Buprenorphine (300 mcg/mL) | Varies widely between species, IM | Varies widely between species | Partial mu opioid agonist |
| Fentanyl | Varies widely between species, IV | Varies widely between species | Full mu opioid agonist |
| Tramadol | Varies widely between species, PO | Varies widely between species | Weak mu opioid agonist Active M1 metabolite Oral bioavailability varies widely between species |
| NSAIDs | |||
| Meloxicam | ~1 mg/kg PO, IM | Varies widely between species | COX-2 selective Efficacy and bioavailability vary widely between species |
| Local Anesthetics** Doses must be calculated carefully due to toxicity potential with overdose (cardiac, CNS, etc.) |
|||
| Liposomal encapsulated bupivacaine (Nocita®) | Up to 0.4 mL/kg intra-incisional infiltration | Up to 3 days | Must cover entire incision due to limited diffusion Favorable safety profile |
| Lidocaine | Up to 6 mg/kg SC | 1-2 hours | Dilution of parent compound is frequently required Usually not recommended due to short duration of action |
| Bupivacaine | Up to 2 mg/kg SC* | 6-8 hours | Dilution of parent compound may be required. Peak onset ~10 minutes |
*Subcutaneous (SC), intramuscular (IM), intravenous (IV), oral (PO).
**Note: lidocaine and bupivacaine/Nocita® should not be mixed together.
Inhalant Anesthetics
Inhalant anesthetic delivery methods:
- Sedation must occur prior to tight-fitting mask induction with inhalant agents; caution should be taken with mask inductions due to personnel exposure, slow induction times and inability to control the airway.
- Agent-specific vaporizers are required, and excess gas must be scavenged.
- Inhalant should be turned off before the mask is removed or the entire procedure performed in a ducted fume hood or biosafety cabinet.
- Anesthetic maintenance using a nosecone or mask is not recommended as endotracheal intubation is easily performed in most species; RARC personnel should be contacted for more information concerning endotracheal intubation in birds.
- A carrier gas is required; usually it is ~100% oxygen.
- Common settings:
- Induction: ~1-2 L/min; maintenance: ~0.5-1 L/min, depending on flow requirements of the system.
- Common settings:
- Isoflurane and sevoflurane can both be used in birds. However, they require separate vaporizers due to different vapor pressures and the MAC values (see Overview of Anesthetic and Analgesia Procedures tab).
- Common vaporizer settings:
- Isoflurane: induction ~2-4%, maintenance ~1-2%
- Sevoflurane: induction ~4-6%, maintenance ~3-4%
- Common vaporizer settings:
- Inhalants are profound cardiovascular and respiratory depressants in birds; premedication is required to smooth induction/recovery and reduce amount of inhalant required.
- Inhalants require proper delivery and controlled ventilation equipment and a scavenging system; RARC personnel can assist in choosing appropriate systems for your laboratory.
Endotracheal Intubation
- See Overview of Anesthetic and Analgesia Procedures tab for additional information.
- Avian endotracheal tube size depends on the species and can range from 22-18 gauge IV catheters to upwards of a 5.0 mm OD in large birds and are often uncuffed due to the presence of complete tracheal rings; damage to the tracheal mucosa is common with excessive cuff pressures.
- Birds produce copious mucous in their airways; small endotracheal tubes can easily become obstructed. Use the largest diameter endotracheal tube possible and continuously monitor end-tidal CO2 levels and waveforms (see below) to quickly diagnose when a mucous plug occurs. Be prepared to re-intubate the bird when necessary.
- Tracheal intubation is relatively simple since the entrance to the trachea is located just at the base of the epiglottis. A lighted laryngoscope can be used to visualize the opening. However, in very small species, the size of the trachea can make the process challenging.
- Place the bird on its keel and open the beak as slight pressure is maintained under the lower beak. The opening can be seen as the bird breathes. Once intubated, the tube can then be taped to the upper beak using transparent tape.
- Birds need to move their keel to breathe – care must be taken to not restrain the bird too tightly during induction or to restrict chest movement during the procedure.
- Birds have significant respiratory depression during anesthetic maintenance with inhalants; controlled ventilation MUST be employed during the entire procedure.
- Please contact RARC if you have questions concerning this procedure.
Anesthetic Monitoring
Anesthetic monitoring techniques:
- Although constant vigilance is required during anesthesia (birds should never be unattended), record vital signs every 5-10 minutes to immediately see values and to assess changes over time. RARC can provide you with an anesthetic record template if needed.
- Vital signs
- Cardiovascular system vital signs:
- Heart rate/pulse rate
- Can be taken using a pulse oximeter (see below), palpation of the heart or stethoscope.
- Normal is upwards of 300 beats/minute, depending on anesthetic agents used and species.
- Arterial blood pressures
- Measured using oscillometric devices or by placement of direct lines into an artery.
- Respiratory system vital signs:
- Respiratory rate
- This can be measured by visualizing the movement of the animal’s keel as it rises and falls or via the end-tidal carbon dioxide monitor (capnometer).
- Normal respiratory rates are species dependent but typically are upwards of 50-100 breaths/min in many small avian species.
- Capnography
- Veterinary-specific monitors are available to measure end-tidal CO2
- Capnometers that depict the CO2 waveforms are essential in birds to detect airway occlusion (“shark fin” waveforms appear).
- Pulse oximetry
- Veterinary-specific monitors are available to measure the amount of oxygen saturation of hemoglobin in blood (normal = >95%) and pulse rate.
- Readings could be impaired by reduced pulsatile strength (hypotension, hypothermia, vasoconstriction, etc.).
- Respiratory rate
- Anesthetic depth
- Palpebral reflexes and limb withdrawal reflexes are performed to determine appropriate anesthetic depth prior to a procedure.
- Body temperature
- Birds can quickly become hypothermic or hyperthermic during anesthesia.
- Rectal thermometers placed in the cloaca or implanted telemeters should be used.
- Temperature should not decrease lower than ~ 98°F throughout the procedure; circulating warm water blankets, forced warm air circulators and warmed anesthetic circuits should be used.
- Excessive surgical plucking and scrubbing and drenching of birds with prepping agents should be minimized.
- Electrical blankets, rice bags, water bottles, and gloves are NOT allowed because they frequently produce burns.
- Pain scoring
- Pain scoring systems tailored to your model should be used and documented in the record.
- Analgesics should be administered as deemed necessary by intervention levels and per protocol or in discussion with the veterinary staff.
- Heart rate/pulse rate
- Cardiovascular system vital signs:
Fluid Therapy
- Intra-operative anesthetic fluid rate should be ~10 mL/kg/hr IV or SC using balanced crystalloid solutions (Plasmalyte, Normosol, Lactated Ringer’s, etc.). Fluid rates and types should be adjusted based on individual animal needs.
- Once fully recovered, supplemental fluid can be given SC. Supplemental fluids should consist of a balanced crystalloid (Plasmalyte, Normosol, Lactated Ringer’s, etc.) and may be supplemented with other substances such as glucose.
Recovery
Factors that contribute to a successful recovery:
- Environment
- Recovery observations must be documented every 5-10 minutes until fully ambulating; a template can be provided by RARC if required.
- Recovery areas should be warm and quiet with dim, yet sufficient lighting to appropriately observe the bird.
- For birds who are hypothermic, recover them in a warmer environment (80°-86°F) such as an incubator. If an incubator is used, it’s imperative to closely monitor them as to avoid hyperthermia.
- Proper hydration and GI function
- Supplemental fluid support (as described above), nutritional support (palatable food, etc.) and/or active feeding techniques may be necessary and beneficial.
- Food intake should be documented and monitored regularly.
Additional Resources
Consult the RARC Veterinary Staff (vet@rarc.wisc.edu) if you have specific questions about the anesthetic procedures included in your IACUC protocol.
For assistance with anesthesia equipment set-up, please contact the RARC Trainers (trainer@rarc.wisc.edu).
Form templates to record anesthesia monitoring are also available on the RARC website.
RARC loans out select anesthesia equipment to the UW-Madison research community for temporary use. Please visit the RARC website to find out what items are available to borrow.
Useful Text Resources:
Veterinary Anesthetic and Monitoring Equipment by Cooley and Johnson
UW-Madison Libraries provides free access to the online version of this textbook.
Veterinary Anesthesia and Analgesia: The Sixth Edition of Lumb and Jones by Lamont et al.
UW-Madison Libraries provides free access to the online version of this textbook.
Exotic Animal Formulary by Carpenter et al.
UW-Madison Libraries provides free access to the online version of this textbook.
Works Cited
“Anesthesia (Guideline).” Anesthesia (Guideline)|Vertebrate Animal Research, https://animal.research.uiowa.edu/iacuc-guidelines-anesthesia.
Danneman PJ and Fish RE. Anesthesia and Analgesia in Laboratory Animals (Second Edition). Academic Press, 2008.
Flecknell PA. Laboratory Animal Anaesthesia Ed. 4. Academic Press, 2015.
Lamont L et al. Veterinary Anesthesia and Analgesia: The Sixth Edition of Lumb and Jones. Wiley Blackwell, 2024.
“Supplemental Anesthesia Training Resource.” Received by Rebecca Johnson, Supplemental Anesthesia Training Resource, 7 May 2020.
RARC’s “Overview of Anesthetic and Analgesic Procedures” must be read prior to accessing the below information.
Contact your RARC veterinarian prior to choosing an anesthetic, including agents that are NOT listed below.
Species-Specific Considerations
Pre-Anesthetic Considerations
- An acclimation period of at least 3 days is required prior to any procedure.
- Cats respond to quiet, gentle, firm handling. A period of 1-2 weeks may be needed to attain positive human-animal interactions and to reduce anxiety.
- Sedation is frequently used to reduce stress of handling. Gabapentin (20-25 mg/kg) orally administered the night before and two hours prior to handling or a procedure is recommended.
- Manual restraint and handling must be kept to a minimum.
- Body weights must be obtained for appropriate drug dosing.
- Cats have small superficial veins for IV access. Thus, many premedications are administered IM. The preferred sites are the lumbar epaxial musculature, but the quadriceps or semimembranosus/semitendinosus muscles may also be used.
- IV catheterization or injection frequently occurs in the cephalic veins although the medial saphenous veins can also be used. For multiple IV injections, implantation of long-term jugular catheters or vascular access ports (VAPS) should be performed.
- IV catheters are usually 20-22 gauge, depending on vein size.
- Food should be withheld for ~ 6-12 hr to reduce regurgitation and/or vomiting. Water should not be withheld.
- Maropitant (1 mg/kg) SC or slow IV may be administered to reduce vomiting.
- Atropine and glycopyrrolate are not routinely administered as premedications. They can be used during anesthesia for vagal-induced arrhythmias.
- Cats may show signs of hyperthermia during anesthesia, especially with agents such as mu-opioids, ketamine, etc. This is particularly seen during recovery. Normothermia should be maintained during any anesthetic procedure to reduce post-anesthetic hyperthermia. Although usually self-limiting, IV fluid therapy, acepromazine administration (vasodilation), and application of fans may reduce hyperthermia.
- Despite anesthetic chosen, sterile ophthalmic ointment must be applied to eyes since blink reflexes are significantly reduced during anesthesia and sedation.
- It is very important to consult your RARC veterinarian when determining what anesthetic is best for your study.
- Kittens require unique considerations; please consult your RARC veterinarian for specific recommendations.
Recommended Agents
Injectable Sedatives, Anesthetics, and Analgesics
Sedatives/Anesthetics
- Examples of the most common sedative and anesthetic agents are included below, along with reversal agents.
- Detailed information concerning agents can be found at: Lamont et al, The Sixth Edition of Lumb & Jones.
- Premedication is common and is administered IM prior to IV catheterization as discussed above.
- Premedication should be done in a familiar environment and cats left in a quiet, dimmed area to achieve maximal sedation.
- Supplemental oxygen should always be administered throughout sedation and/or anesthesia.
Sedatives Used in Cats
| Drug | Dosage/Route* | Duration | Comments |
| Acepromazine | 0.01-0.03 mg/kg IM, IV | 45-60 minutes | Mild sedation only Vasodilation and hypotension |
| Midazolam | 0.1-0.2 mg/kg IM, IV | 30-60 minutes | NEVER administered alone Mild to no sedation unless co-administered with another agent Used for muscle relaxation Reversed with flumazenil |
| Dexmedetomidine | 0.001-0.01 mg/kg IM, IV | 30-60 minutes | Mild to profound sedation, based on dose Some analgesia Reversed with atipamezole |
*Intramuscular (IM), intravenous (IV). These can be used with other agents (such as opioids and dissociatives) to produce analgesia, profound sedation or general anesthesia as below.
Injectable Anesthetics Used in Cats
| Drug | Dosage/Route* | Anesthetic Duration | Comments |
| Dissociative Combinations | |||
| Ketamine + midazolam |
5-10 mg/kg ketamine + 0.1-0.2 mg/kg midazolam IM |
Up to 30 minutes | Light anesthetic plane Minimal analgesia - frequently used in combination with an opioid Useful for non-invasive procedures Midazolam reversed with flumazenil |
| Ketamine + dexmedetomidine |
5-10 mg/kg ketamine + 0.005-0.01 mg/kg dexmedetomidine IM |
Up to 60 minutes | Light to moderate anesthetic plane Cardiorespiratory depression; oxygen supplementation is recommended Dexmedetomidine reversed with atipamezole |
| Others | |||
| Alfaxalone | 0.5-3 mg/kg IM, IV | 30-40 minutes | NEVER administered alone Requires a muscle relaxant (midazolam, opioid, etc.) Moderate to profound sedation to anesthesia Oxygenation supplementation is recommended |
| Propofol | 1-4 mg/kg IV slow bolus 0.2-0.8 mg/kg/min IV constant rate infusion (CRI) |
5-7 minutes | Titrate to effect for anesthetic induction or used as a CRI as part of partial intravenous anesthesia (PIVA) Cardiorespiratory depression; oxygen supplementation is recommended Apnea is common |
*Intramuscular (IM), intravenous (IV). These can be used with opioids to produce analgesia with profound sedation or general anesthesia as below.
Reversal Agents Used in Cats
| Drug | Dosage/Route* | Reversal Category | Reversal for: |
| Atipamexole | 0.05-0.2 mg/kg SC, IM | Alpha-2 adrenergic receptors | Dexmedetomidine |
| Flumazenil | 0.02-0.08 mg/kg SC, IM, IV | Benzodiazepines | Midazolam |
| Naloxone | 0.1-1 mg/kg SC, IM, IV | Opioids | Buprenorphine, hydromorphone, etc. |
*Subcutaneous (SC), intramuscular (IM), intravenous (IV)
Analgesics
- Examples of common analgesic agents are listed below. Others are available; please contact RARC veterinarians to discuss alternatives such as gabapentin, tramadol, etc.
- Unrelieved pain has profound physiologic consequences, which may alter research results.
- Pain assessment in cats consists of evaluating behavioral and physiologic parameters and use of validated pain scoring systems such as the Unesp-Botucatu Feline Pain Scale – Short Form (UFEPS-SF) (Luna et al 2022).
- Feline pain scales include abnormal gait and posture, eye and lid position, biting or licking the painful area, tail movement, attentive or restless behavior, and reaction to palpation of the affected area. The pain score and other signs must be documented in the record along with any analgesic intervention taken.
- The IACUC requires the use of preemptive analgesia (analgesics given prior to the first skin incision) for all survival surgical procedures unless scientifically justified.
- Pre-emptive analgesics may decrease the amount of required anesthetic drugs; doses of both injectable and inhalant agents should be adjusted accordingly.
Analgesic Agents Used in Cats
| Drug | Dosage/Route* | Frequency | Comments |
| Opioids/Sodium Channel Blockers/NMDA Antagonists | |||
| Buprenorphine (300 mcg/mL) | 0.02-0.05 mg/kg IM, IV, TM | 6-8 hours | Partial mu-opioid agonist Moderate analgesia |
| High concentration buprenorphine (Simbadol™; 1.8 mg/mL) | 0.18-0.24 mg/kg SC | 24-72 hours | Partial mu-opioid agonist Moderate analgesia |
| Hydromorphone | 0.1-0.2 mg/kg IM, IV | 2-4 hours | Full mu opioid agonist Profound analgesia |
| Butorphanol | 0.2-0.5 mg/kg IM, IV | 1-2 hours | Mu opioid antagonist; kappa opioid agonist Mild, short analgesia |
| Fentanyl | 2-5 mcg/kg bolus IV 2-20 mcg/kg/hr IV OR 1-2 mcg/kg/hr TD |
CRI OR Patch |
Full mu agonist Profound analgesia Patch takes up to 18 hrs for full effect and can be variable; up to 72 hr duration |
| Methadone | 0.1-0.5 mg/kg IM, IV | ~4 hours | Full mu agonist and NMDA antagonist Profound analgesia |
| Ketamine | 0.25-1 mg/kg bolus SC, IV 2-20 mcg/kg/min IV |
CRI | Excellent somatic analgesia Reduces inhalant required |
| NSAIDs | |||
| Meloxicam | 0.1 mg/kg SC, PO | 24 hours | COX-2 selective Can titrate down to 0.05 mg/kg PO every other day for chronic use |
| Robenacoxib (Onsior®) | 1 mg/kg PO; 2 mg/kg SC OR 2.5 - 6 kg BW: 1 whole tablet PO (6 mg) 6.1 - 12 kg BW: 2 whole tables PO (12 mg) |
24 hours | COX-2 selective Use up to 3 days Inaccurate dosing if < 2.5kg BW PO tables cannot be broken Give PO dose with food |
| Local Anesthetics** | |||
| Liposomal encapsulated bupivacaine (Nocita®) | Up to 0.4 mL/kg intra-incisional infiltration | Up to 3 days | Must cover entire incision due to limited diffusion Favorable safety profile |
| Lidocaine | Up to 6 mg/kg SC | 1-2 hours | Usually not recommended due to short duration of action |
| Bupivacaine | Up to 2 mg/kg SC | 6-8 hours | Peak onset ~10 minutes |
*Subcutaneous (SC), intramuscular (IM), intravenous (IV), oral (PO), transmucosal (TM), transdermal (TD).
**Note: lidocaine and bupivacaine/Nocita® should not be mixed together.
Inhalant Anesthetics
Inhalant anesthetic delivery methods:
- Mask or chamber induction is not used due to personnel exposure, slow induction times and inability to control the airway.
- Anesthetic maintenance using a mask can be performed but is not recommended due to personnel exposure, inability to protect the airway from regurgitation, and inability to control ventilation.
- Endotracheal intubation is recommended during anesthetic maintenance; RARC personnel should be contacted for more information concerning endotracheal intubation in cats.
- A carrier gas is required; usually it is ~100% oxygen.
- Common settings:
- Immediately following induction: ~2 L/min; maintenance: ~1 L/min.
- Common settings:
- Isoflurane and sevoflurane can both be used in cats. However, they require separate vaporizers due to different vapor pressures and the MAC values (see Overview of Anesthetic and Analgesia Procedures tab).
- Common vaporizer settings:
- Isoflurane: immediately following induction ~2-4%, maintenance ~1-2%
- Sevoflurane: immediately following induction ~4-6%, maintenance ~3-4%
- Common vaporizer settings:
- Inhalants are profound cardiovascular and respiratory depressants; premedication is required to reduce amount of inhalant required.
- Inhalants require proper circle/rebreathing and scavenging systems; RARC personnel can assist in choosing appropriate systems for your laboratory.
- For small cats (<3 kg), a non-rebreathing system must be used to reduce resistance to breathing.
Endotracheal Intubation
- See Overview of Anesthetic and Analgesia Procedures tab for additional information. Please consult with RARC personnel if you are performing tracheal intubation in cats.
- Adult cat endotracheal tubes are usually 3.5-5.0 mm; kittens require much smaller tubes and depending on age/size can be a small as 2.0 mm.
- Laryngoscopes are used to ensure proper visualization of the larynx for atraumatic intubation. Fiberoptic laryngoscope blades should be used.
- Cats should be placed in ventral recumbency with the neck extended. The laryngoscope blade should be placed on the base of the tongue – not on the epiglottis – to visualize the trachea. 2% lidocaine (~ 0.1 mL in an adult) is placed on the arytenoid cartilages. The endotracheal tube cuff should be lubricated with a sterile, water-soluble lubricant to form an appropriate seal. The endotracheal tube is gently placed curved side down through the glottis and the cuff inflated only to an airway pressure of ~ 20 cm H2O and no higher using the minimal occlusion pressure technique.
- Local anesthetics with benzocaine may induce methemoglobinemia in cats – DO NOT USE!
- DO NOT simply put air in the cuff – ONLY inflate it to seal at 20 cm H2
- Gentle techniques must be used; trauma is common and laryngeal/tracheal rupture can occur. Pneumomediastinum and pneumothorax may result from overzealous intubation attempts.
- Supraglottic airway devices (V-Gel®) have been used in cats. However, they do not form a complete seal and MUST be used with a capnograph to observe correct placement and reduce obstruction. Contact RARC regarding this procedure.
- Please contact RARC if you have questions concerning this procedure.
Anesthetic Monitoring
Anesthetic monitoring techniques:
- Although constant vigilance is required during anesthesia (cats should never be unattended), record vital signs every 5-10 minutes to immediately see values and to assess changes over time. RARC can provide you with an anesthetic record template if needed.
- Vital signs
- Cardiovascular system vital signs:
- Heart rate/pulse rate
- Can be taken using a pulse oximeter (see below), palpation of the heart or stethoscope.
- Normal ~120-200 beats/minute, depending on anesthetic agents used.
- Arterial blood pressures
- Measured using oscillometric devices or by placement of direct lines into an artery.
- Mean arterial pressure should always be kept above ~ 70 mmHg to ensure organ perfusion.
- Heart rate/pulse rate
- Respiratory system vital signs:
- Respiratory rate
- This can be measured by visualizing the movement of the animal’s chest wall as it rises and falls or via the end-tidal carbon dioxide monitor (capnometer).
- Normal respiratory rates typically are ~20-40 breaths/min.
- Capnography
- Veterinary-specific monitors measure end-tidal CO2 levels and produce capnograph waves. These are useful in detecting abnormalities in ventilation and appropriate tracheal intubation.
- Values will read artificially low if a non-rebreathing circuit is used.
- Normal levels are ~30-35 mmHg.
- Pulse oximetry
- Veterinary-specific monitors are available to measure the amount of oxygen saturation of hemoglobin in blood (normal = >95%) and pulse rate.
- Readings could be impaired by reduced pulsatile strength (hypotension, hypothermia, vasoconstriction, etc.).
- Respiratory rate
- Anesthetic depth
- Jaw tone, palpebral reflexes and limb withdrawal reflexes are performed to determine appropriate anesthetic depth prior to a procedure.
- Body temperature
- Cats can quickly become hypothermic or hyperthermic during anesthesia (normal = 100.5-102.5 °F).
- Rectal or esophageal thermometers or implanted telemeters should be used.
- Temperature should not decrease lower than ~ 98°F throughout the procedure; circulating warm water blankets, forced warm air circulators and warmed anesthetic circuits should be used.
- Surgical clipping and scrubbing or prepping agents should be minimized; cats should not be “soaked” with solutions.
- Electrical blankets, rice bags, water bottles, and gloves are NOT allowed because they frequently produce burns.
- Pain scoring
- Cardiovascular system vital signs:
- Validated pain scoring systems such as the the Unesp-Botucatu Feline Pain Scale – Short Form (UFEPS-SF) (Luna et al 2022) must be used and documented in the record.
- Analgesics must be administered as deemed necessary by intervention levels and per protocol or in discussion with RARC veterinary personnel.
Fluid Therapy
- Intra-operative anesthetic fluid rate should be ~3 mL/kg/hr IV using balanced crystalloid solutions (Plasmalyte, Normosol, Lactated Ringer’s, etc.). Fluid rates and types should be adjusted based on individual animal needs.
- Voluntary fluid intake should be documented after the procedure into the recovery period.
- Once fully recovered, supplemental fluid can be given IV or SC if required. Supplemental fluids should consist of a balanced crystalloid (Plasmalyte, Normosol, Lactated Ringer’s, etc.) and may be supplemented with other substances such as glucose (not SC).
Recovery
Factors that contribute to a successful recovery:
- Environment
- Recovery observations must be documented every 5-10 minutes until fully ambulating; a template can be provided by RARC if required.
- Recovery areas should be warm and quiet with dim, yet sufficient lighting to appropriately observe the cats.
- If appropriate room temperatures cannot be achieved, the incorporation of supplemental heat sources should be used as above.
- Cats should be extubated at the first sign of tongue or ear movement to reduce laryngospasm. The return of the swallow reflex and ability to maintain sternal recumbency must be ensured.
- Proper hydration and GI function
- Supplemental fluid support (as described above), nutritional support (moist food, enriched food, etc.) and/or active feeding techniques may be necessary and beneficial. Palatable food should be offered when cats are ambulating and have fully regained their swallow reflex to reduce GI stasis.
- Like fluid intake, food intake should be documented in the record and monitored regularly.
Cat Validated Pain Scoring System
Pain scale: Unesp-Botucatu Feline Pain Scale
| ITEM | Description | Score |
| Evaluate the cat's posture in the cage for 2 minutes: | ||
| 1 | Natural, relaxed, and/or moves normally | 0 |
| Natural but tense, does not move or moves little or is reluctant to move | 1 | |
| Hunched position and/or dorso-lateral recumbency | 2 | |
| Frequently changes position or restless | 3 | |
| Check which of the following apply: | ||
| 2 | The cat contracts and extends its pelvic limbs and/or contracts its abdominal muscles (flank) | |
| The cats' eyes are partially closed (do not consider this item present until 1 hr after the end of anesthesia) | ||
| The cat licks and/or bites the painful site | ||
| The cat moves its tail strongly | ||
| SCORING: All above behaviors in Item 2 are absent | 0 | |
| Presence of one of the above behaviors in Item 2 | 1 | |
| Presence of two of the above behaviors in Item 2 | 2 | |
| Presence of three or all of the above behaviors in Item 2 | 3 | |
| Evaluation of comfort, activity, and attitude after the cage is open and how attentive the cat is to the observer and/or surroundings: | ||
| 3 | Comfortable and attentive | 0 |
| Quiet and slightly attentive | 1 | |
| Quiet and not attentive. The cat may face the back of the cage. | 2 | |
| Uncomfortable, restless, and slightly attentive or not attentive. The cat may face the back of the cage. | 3 | |
| Evaluation of the cat's reaction when touching, followed by pressuring around the painful site: | ||
| 4 | Does not react. | 0 |
| Does not react when the painful site is touched but does react when it is gently pressed. | 1 | |
| Reacts when the painful site is touched and when pressed. | 2 | |
| Does not allow touch or palpation. | 3 | |
Scale and Decision for Analgesic:
- Score total can range from 0 to 12
- Analgesic intervention given at a score ≥ 4
Additional Resources
Consult the RARC Veterinary Staff (vet@rarc.wisc.edu) if you have specific questions about the anesthetic procedures included in your IACUC protocol.
For assistance with anesthesia equipment set-up, please contact the RARC Trainers (trainer@rarc.wisc.edu).
Form templates to record anesthesia monitoring are also available on the RARC website.
RARC loans out select anesthesia equipment to the UW-Madison research community for temporary use. Please visit the RARC website to find out what items are available to borrow.
Useful Text Resources:
Veterinary Anesthetic and Monitoring Equipment by Cooley and Johnson
UW-Madison Libraries provides free access to the online version of this textbook.
Veterinary Anesthesia and Analgesia: The Sixth Edition of Lumb and Jones by Lamont et al.
UW-Madison Libraries provides free access to the online version of this textbook.
Works Cited
“Anesthesia (Guideline).” Anesthesia (Guideline)|Vertebrate Animal Research, https://animal.research.uiowa.edu/iacuc-guidelines-anesthesia.
Danneman PJ and Fish RE. Anesthesia and Analgesia in Laboratory Animals (Second Edition). Academic Press, 2008.
Flecknell PA. Laboratory Animal Anaesthesia Ed. 4. Academic Press, 2015.
Lamont L et al. Veterinary Anesthesia and Analgesia: The Sixth Edition of Lumb and Jones. Wiley Blackwell, 2024.
Luna SPL, Trindade PHE, Monteiro BP, Crosignani N, della Rocca G, Ruel HLM, Yamashita K, Kronen P, Tseng CT, Teixeira L, Steagall PV. 2022. Multilingual validation of the short form of the Unesp-Botucatu Feline Pain Scale (UFEPS-SF) PeerJ 10:e13134 https://doi.org/10.7717/peerj.13134
“Supplemental Anesthesia Training Resource.” Received by Rebecca Johnson, Supplemental Anesthesia Training Resource, 7 May 2020.
RARC’s “Overview of Anesthetic and Analgesic Procedures” must be read prior to accessing the below information.
Contact your RARC veterinarian prior to choosing an anesthetic, including agents that are NOT listed below.
Species-Specific Considerations
Pre-Anesthetic Considerations
- An acclimation period of at least 3 days is required prior to any procedure.
- Dogs respond to quiet, gentle, firm handling. A period of 1-2 weeks may be needed to attain positive human-animal interactions and to reduce anxiety.
- Sedation is frequently used to reduce stress of handling. Gabapentin (20-25 mg/kg) and trazodone (5-10 mg/kg) orally administered the night before and two hours prior to handling or a procedure is recommended.
- Manual restraint and handling must be kept to minimum.
- Body weights must be obtained for appropriate drug dosing.
- Premedications are frequently administered IM. The preferred site is the lumbar epaxial musculature, but the quadriceps or semimembranosus/semitendinosus muscles may also be used.
- IV catheterization or injection frequently occurs in the cephalic veins although the lateral saphenous veins can also be used. For multiple IV injections, implantation of long-term jugular catheters or vascular access ports (VAPS) should be performed.
- IV catheters are usually 18-20 gauge, depending on vein size.
- Food should be withheld for ~ 6-12 hr to reduce regurgitation and/or vomiting. Water should not be withheld.
- Maropitant (1 mg/kg SC or slow IV or 2 mg/kg PO) may be administered to reduce vomiting.
- Atropine and glycopyrrolate are not routinely administered as premedications. They can be used during anesthesia for vagal-induced arrhythmias.
- Despite anesthetic chosen, sterile ophthalmic ointment must be applied to eyes since blink reflexes are significantly reduced during anesthesia and sedation.
- It is very important to consult your RARC veterinarian when determining what anesthetic is best for your study.
- Puppies require unique considerations; please consult your RARC veterinarian for specific recommendations.
Recommended Agents
Injectable Sedatives, Anesthetics, and Analgesics
Sedatives/Anesthetics
- Examples of sedative and anesthetic agents are included below, along with reversal agents.
- Detailed information concerning agents can be found at: Lamont et al, The Sixth Edition of Lumb & Jones.
- Premedication is common and is administered IM prior to IV catheterization as discussed above.
- Premedication should be done in a familiar environment and dogs left in a quiet, dimmed area to achieve maximal sedation.
- Supplemental oxygen should always be administered throughout sedation and/or anesthesia.
Sedatives Used in Dogs
| Drug | Dosage/Route* | Duration | Comments |
| Acepromazine | 0.005-0.03 mg/kg IM, IV | 45-60 minutes | Mild sedation only Vasodilation and hypotension |
| Midazolam | 0.1-0.5 mg/kg IM, IV | 30-60 minutes | NEVER administered alone Mild to no sedation unless co-administered with another agent Used for muscle relaxation Reversed with flumazenil |
| Dexmedetomidine | 0.001-0.01 mg/kg IM, IV | 30-60 minutes | Mild to profound sedation, based on dose Some analgesia Reversed with atipamezole |
*Intramuscular (IM), intravenous (IV). These can be used with other agents (such as opioids) to produce analgesia, profound sedation or general anesthesia as below.
Injectable Anesthetics Used in Dogs
| Drug | Dosage/Route* | Anesthetic Duration | Comments |
| Alfaxalone | 0.5-3 mg/kg IM, IV | 30-40 minutes | NEVER administered alone Requires a muscle relaxant (midazolam, opioid, etc.) when administered IM Moderate to profound sedation to anesthesia Oxygenation supplementation is recommended |
| Ketamine | 2-5 mg/kg IV | ~10 minutes | Requires a muscle relaxant (midazolam) to produce general anesthesia IV; not routinely administered IM in dogs Oxygen supplementation is recommended |
| Propofol | 1-4 mg/kg IV slow bolus 0.2-0.8 mg/kg/min IV constant rate infusion (CRI) |
5-7 minutes | Titrate to effect for anesthetic induction or used as a CRI as part of partial intravenous anesthesia (PIVA) Cardiorespiratory depression; oxygen supplementation is recommended Apnea is common |
*Intramuscular (IM), intravenous (IV). These can be used with opioids to produce analgesia with profound sedation or general anesthesia as below.
Reversal Agents Used in Dogs
| Drug | Dosage/Route* | Reversal Category | Reversal for: |
| Atipamezole | 0.05-0.2 mg/kg SC, IM | Alpha-2 adrenergic receptors | Dexmedetomidine |
| Flumazenil | 0.02-0.08 mg/kg SC, IM, IV | Benzodiazepines | Midazolam |
| Naloxone | 0.1-1 mg/kg SC, IM, IV | Opioids | Buprenorphine, hydromorphone, etc. |
*Subcutaneous (SC), intramuscular (IM), intravenous (IV)
Analgesics
- Examples of common analgesic agents are listed below. Others are available; please contact RARC veterinarians to discuss alternatives such as gabapentin, etc.
- Unrelieved pain has profound physiologic consequences, which may alter research results.
- Pain assessment in dogs consists of evaluating behavioral and physiologic parameters and use of validated pain scoring systems such as the short form of the Glascow Composite Measure Pain Scale (CMPS-SF) (Reid et al 2007).
- Canine pain scales include abnormal gait and posture, demeanor, biting, rubbing or licking the painful area, attentive or restless behavior, and reaction to palpation of the affected area. The pain score and other signs must be documented in the record along with any analgesic intervention taken.
- The IACUC requires the use of preemptive analgesia (analgesics given prior to the first skin incision) for all survival surgical procedures unless scientifically justified.
- Pre-emptive analgesics may decrease the amount of required anesthetic drugs; doses of both injectable and inhalant agents should be adjusted accordingly.
Analgesic Agents Used in Dogs
| Drug | Dosage/Route* | Frequency | Comments |
| Opioids/NMDA Antagonists | |||
| Buprenorphine (300 mcg/mL) | 0.02-0.05 mg/kg IM, IV | ~6 hours | Partial mu-opioid agonist Mild analgesia |
| Hydromorphone | 0.1-0.2 mg/kg IM, IV | 2-4 hours | Full mu opioid agonist Profound analgesia |
| Butorphanol | 0.2-0.5 mg/kg IM, IV | 1-2 hours | Mu opioid antagonist; kappa opioid agonist Mild, short analgesia |
| Fentanyl |
2-5 mcg/kg bolus IV |
CRI OR Patch |
Full mu agonist Profound analgesia Patch takes up to 18 hours for full effect and can be variable; up to 72 hour duration |
| Methadone | 0.1-0.5 mg/kg IM, IV | ~4 hours | Full mu agonist and NMDA antagonist Profound analgesia |
| Ketamine | 0.25-1 mg/kg bolus SC, IV 2-20 mcg/kg/min IV |
CRI | NMDA antagonist Excellent somatic analgesia Reduces inhalant required |
| NSAIDs | |||
| Meloxicam | 0.1 mg/kg SC, PO | 24 hours | COX-2 selective Can titrate down to 0.05 mg/kg PO every other day for chronic use |
| Carprofen | 2.2-4.4 mg/kg SC, PO | 12 hours (2.2 mg/kg) - 24 hours (4.4 mg/kg) | COX-2 selective |
| Local Anesthetics** | |||
| Liposomal encapsulated bupivacaine (Nocita®) | Up to 0.4 mL/kg intra-incisional infiltration | Up to 3 days | Must cover entire incision due to limited diffusion Favorable safety profile |
| Lidocaine | Up to 6 mg/kg SC | 1-2 hours | Usually not recommended due to short duration of action |
| Bupivacaine | Up to 2 mg/kg SC | 6-8 hours | Peak onset ~10 minutes |
*Subcutaneous (SC), intramuscular (IM), intravenous (IV), oral (PO), transdermal (TD).
**Note: lidocaine and bupivacaine/Nocita® should not be mixed together.
Inhalant Anesthetics
Inhalant anesthetic delivery methods:
- Mask or chamber induction is not used due to personnel exposure, slow induction times and inability to control the airway.
- Anesthetic maintenance using a mask is not recommended due to personnel exposure, inability to protect the airway from regurgitation, and inability to control ventilation.
- Endotracheal intubation is required during anesthetic maintenance; RARC personnel should be contacted for more information concerning endotracheal intubation in dogs.
- A carrier gas is required; usually it is ~100% oxygen.
- Common settings:
- Immediately following induction: ~2 L/min; maintenance: ~1 L/min.
- Common settings:
- Isoflurane and sevoflurane can both be used in dogs. However, they require separate vaporizers due to different vapor pressures and the MAC values (see Overview of Anesthetic and Analgesia Procedures tab).
- Common vaporizer settings:
- Isoflurane: immediately following induction ~2-3%, maintenance ~1-2%
- Sevoflurane: immediately following induction ~3-4%, maintenance ~2-3%
- Common vaporizer settings:
- Inhalants are profound cardiovascular and respiratory depressants; premedication is required to reduce amount of inhalant required.
- Inhalants require proper circle/rebreathing and scavenging systems; RARC personnel can assist in choosing appropriate systems for your laboratory.
Endotracheal Intubation
- See Overview of Anesthetic and Analgesia Procedures tab for additional information. Please consult with RARC personnel if you are performing tracheal intubation in dogs.
- Adult dog endotracheal tubes are usually 7.0-12.0 mm OD; puppies require much smaller tubes and depending on age/size can be a small as 4.0 mm OD.
- Laryngoscopes are used to ensure proper visualization of the larynx for atraumatic intubation. Fiberoptic laryngoscope blades should be used.
- Dogs should be placed in ventral recumbency with the neck extended. The laryngoscope blade should be placed on the base of the tongue – not on the epiglottis – to visualize the trachea. The endotracheal tube cuff should be lubricated with a sterile, water-soluble lubricant to form an appropriate seal. The endotracheal tube is gently placed curved side down through the glottis and the cuff inflated only to an airway pressure of ~ 20 cm H2O and no higher using the minimal occlusion pressure technique.
- DO NOT simply put air in the cuff – ONLY inflate it to seal at 20 cm H2
- Supraglottic airway devices (V-Gel®) have been used in dogs. However, they do not form a complete seal and MUST be used with a capnograph to observe correct placement and reduce obstruction. Contact RARC regarding this procedure.
- Please contact RARC if you have questions concerning this procedure.
Anesthetic Monitoring
Anesthetic monitoring techniques:
- Although constant vigilance is required during anesthesia (dogs should never be unattended), record vital signs every 5-10 minutes to immediately see values and to assess changes over time. RARC can provide you with an anesthetic record template if needed.
- Vital signs
- Cardiovascular system vital signs:
- Heart rate/pulse rate
- Can be taken using a pulse oximeter (see below), palpation of the heart or stethoscope.
- Normal ~70-120 beats/minute, depending on anesthetic agents used. Slower rates are associated with the use of alpha-2 agonists (~50-60 beats/minute).
- Arterial blood pressures
- Measured using oscillometric devices or by placement of direct lines into an artery.
- Mean arterial pressure should always be kept above ~70 mmHg to ensure organ perfusion.
- Heart rate/pulse rate
- Respiratory system vital signs:
- Respiratory rate
- This can be measured by visualizing the movement of the animal’s chest wall as it rises and falls or via the end-tidal carbon dioxide monitor (capnometer).
- Normal respiratory rates typically are ~20-40 breaths/min awake and 10-20 breaths/min when sedated/anesthetized.
- Capnography
- Veterinary-specific monitors measure end-tidal CO2 levels and produce capnograph waves. These are useful in detecting abnormalities in ventilation and appropriate tracheal intubation.
- Normal levels are ~35-45 .
- Pulse oximetry
- Veterinary-specific monitors are available to measure the amount of oxygen saturation of hemoglobin in blood (normal = >95%) and pulse rate.
- Readings could be impaired by reduced pulsatile strength (hypotension, hypothermia, vasoconstriction, etc.).
- Respiratory rate
- Anesthetic depth
- Jaw tone, palpebral reflexes and limb withdrawal reflexes are performed to determine appropriate anesthetic depth prior to a procedure.
- Body temperature
- Dogs can quickly become hypothermic or, less often, hyperthermic during anesthesia (normal = 100.5-102.5 °F).
- Rectal or esophageal thermometers or implanted telemeters should be used.
- Temperature should not decrease lower than ~ 98°F throughout the procedure; circulating warm water blankets, forced warm air circulators and warmed anesthetic circuits should be used.
- Surgical clipping and scrubbing or prepping agents should be minimized; dogs should not be “soaked” with solutions.
- Electrical blankets, rice bags, water bottles, and gloves are NOT allowed because they frequently produce burns.
- Pain scoring
- Validated pain scoring systems such as the short form of the Glascow Composite Measure Pain Scale (CMPS-SF) (Reid et al 2007) must be used and documented in the record.
- Analgesics must be administered as deemed necessary by intervention levels and per protocol or in discussion with RARC veterinary personnel.
- Validated pain scoring systems such as the short form of the Glascow Composite Measure Pain Scale (CMPS-SF) (Reid et al 2007) must be used and documented in the record.
- Cardiovascular system vital signs:
Fluid Therapy
- Intra-operative anesthetic fluid rate should be ~5 mL/kg/hr IV using balanced crystalloid solutions (Plasmalyte, Normosol, Lactated Ringer’s, etc.). Fluid rates and types should be adjusted based on individual animal needs.
- Voluntary fluid intake should be documented after the procedure into the recovery period.
- Once fully recovered, supplemental fluid can be given IV or SC if required. Supplemental fluids should consist of a balanced crystalloid (Plasmalyte, Normosol, Lactated Ringer’s, etc.) and may be supplemented with other substances such as glucose (not SC).
Recovery
Factors that contribute to a successful recovery:
- Environment
- Recovery observations must be documented every 5-10 minutes until fully ambulating; a template can be provided by RARC if required.
- Recovery areas must be warm and quiet with dim, yet sufficient lighting to appropriately observe the dogs.
- If appropriate room temperatures cannot be achieved, the incorporation of supplemental heat sources should be used as above.
- Dogs are recovered in sternal recumbency with their heads elevated to reduce aspiration of esophageal/stomach contents.
- Dogs are extubated at the return of the swallow reflex and the ability to maintain sternal recumbency must be ensured.
- Proper hydration and GI function
- Supplemental fluid support (as described above), nutritional support (moist food, enriched food, etc.) and/or active feeding techniques may be necessary and beneficial. Palatable food should be offered when dogs are ambulating and have fully regained their swallow reflex to reduce GI stasis.
- Like fluid intake, food intake must be documented in the record and monitored regularly.
Dog Validated Pain Scoring System
Pain scale: Glasgow Composite Measure Pain Score
| ITEM | Description | Score |
| Evaluate the dog in the kennel. The dog is... | ||
| 1 | Quiet | 0 |
| Crying or whimpering | 1 | |
| Groaning | 2 | |
| Screaming | 3 | |
| 2 |
Ignoring any wound or painful area | 0 |
| Looking at wound or painful area | 1 | |
| Licking wound or painful area | 2 | |
| Rubbing wound or painful area | 3 | |
| Chewing wound or painful area | 4 | |
| Evaluate the dog on a lead and out of the kennel. When the dog rises/walks, it is... | ||
| 3 |
Normal | 0 |
| Lame | 1 | |
| Slow or reluctant | 2 | |
| Stiff | 3 | |
| Refusing to move | 4 | |
| Evaluate the dog's reaction to applying gentle pressure to the area ~2 inches around the wound or painful area. The dog... | ||
| 4 |
Does not react. | 0 |
| Looks around. | 1 | |
| Flinches. | 2 | |
| Growls or guards the area. | 3 | |
| Snaps. | 4 | |
| Cries. | 5 | |
| Overall, the dog is... | ||
| 5 | Happy and content or happy and bouncy | 0 |
| Quiet | 1 | |
| Indifferent or non-responsive to surroundings | 2 | |
| Nervous or anxious or fearful | 3 | |
| Depressed or non-responsive to stimulation | 4 | |
| 6 | Comfortable | 0 |
| Unsettled | 1 | |
| Restless | 2 | |
| Hunched or tense | 3 | |
| Rigid | 4 | |
Scale and Decision for Analgesic:
- Score total can range from 0 to 24
- Analgesic intervention given at a score >5
Additional Resources
Consult the RARC Veterinary Staff (vet@rarc.wisc.edu) if you have specific questions about the anesthetic procedures included in your IACUC protocol.
For assistance with anesthesia equipment set-up, please contact the RARC Trainers (trainer@rarc.wisc.edu).
Form templates to record anesthesia monitoring are also available on the RARC website.
RARC loans out select anesthesia equipment to the UW-Madison research community for temporary use. Please visit the RARC website to find out what items are available to borrow.
Useful Text Resources:
Veterinary Anesthetic and Monitoring Equipment by Cooley and Johnson
UW-Madison Libraries provides free access to the online version of this textbook.
Veterinary Anesthesia and Analgesia: The Sixth Edition of Lumb and Jones by Lamont et al.
UW-Madison Libraries provides free access to the online version of this textbook.
Works Cited
“Anesthesia (Guideline).” Anesthesia (Guideline)|Vertebrate Animal Research, https://animal.research.uiowa.edu/iacuc-guidelines-anesthesia.
Danneman PJ and Fish RE. Anesthesia and Analgesia in Laboratory Animals (Second Edition). Academic Press, 2008.
Flecknell PA. Laboratory Animal Anaesthesia Ed. 4. Academic Press, 2015.
Lamont L et al. Veterinary Anesthesia and Analgesia: The Sixth Edition of Lumb and Jones. Wiley Blackwell, 2024.
Reid J et al. "Development of the short-form Glasgow Composite Measure Pain Scale (CMPS-SF) and derivation of an analgesic intervention score." Animal welfare 16.S1 (2007): 97-104. (available at https://www.newmetrica.com/wp-content/uploads/2016/09/Reid-et-al-2007.pdf )
“Supplemental Anesthesia Training Resource.” Received by Rebecca Johnson, Supplemental Anesthesia Training Resource, 7 May 2020.
RARC’s “Overview of Anesthetic and Analgesic Procedures” must be read prior to accessing the below information.
Contact your RARC veterinarian prior to choosing an anesthetic, including agents that are NOT listed below.
Species-Specific Considerations
Pre-Anesthetic Considerations
- An acclimation period of at least 3 days is required prior to any procedure.
- Ferrets vary in temperament – approach quietly and gently. A period of 1-2 weeks may be needed to attain positive human-animal interactions and to reduce anxiety.
- Support both their shoulders by their back and their hind quarters while handling.
- Body weights must be obtained for appropriate drug dosing.
- Ferrets have small superficial veins for IV access. Thus, many premedications are administered IM. The preferred sites are the lumbar epaxial musculature, but the biceps femoris can also be used.
- IV catheterization or injection frequently occurs in the cephalic veins; although, the lateral saphenous veins can also be used. For multiple blood samples, the jugular vein or cranial vena cava can be used but usually require sedation or general anesthesia.
- IV catheters are usually 22-24 gauge, depending on vein size.
- Food should be withheld for ~ 6-12 hr to reduce regurgitation and aspiration. Water should not be withheld.
- Atropine and glycopyrrolate are not routinely administered as premedications. They can be used during anesthesia for vagal-induced arrhythmias.
- Ferrets frequently shiver during handling and anesthesia.
- Despite anesthetic chosen, sterile ophthalmic ointment must be applied to eyes since blink reflexes are significantly reduced during anesthesia and sedation.
- It is very important to consult your RARC veterinarian when determining what anesthetic is best for your study.
- Kits require unique considerations; please consult your RARC veterinarian for specific recommendations.
Recommended Agents
Injectable Sedatives, Anesthetics, and Analgesics
Sedatives/Anesthetics
- Examples of sedative and anesthetic agents are included below, along with reversal agents.
- Detailed information concerning agents can be found at: Lamont et al, The Sixth Edition of Lumb & Jones.
- Premedication is common and is administered IM prior to IV catheterization as discussed above.
- Premedication should be done in a familiar environment and ferrets left in a quiet, dimmed area to achieve maximal sedation.
- Supplemental oxygen should always be administered throughout sedation and/or anesthesia.
Sedatives Used in Ferrets
| Drug | Dosage/Route* | Duration | Comments |
| Acepromazine | 0.03-0.05 mg/kg IM, IV | 45-60 minutes | Mild sedation only Vasodilation and hypotension |
| Midazolam | 0.2-0.5 mg/kg IM, IV | 30-60 minutes | NEVER administered alone Mild to no sedation unless co-administered with another agent Used for muscle relaxation Reversed with flumazenil |
| Dexmedetomidine | 0.01-0.02 mg/kg IM, IV | 30-60 minutes | Mild to profound sedation, based on dose Some analgesia Reversed with atipamezole |
*Intramuscular (IM), intravenous (IV). These can be used with other agents (such as opioids and dissociatives) to produce analgesia, profound sedation, or general anesthesia as below.
Injectable Anesthetics Used in Ferrets
| Drug | Dosage/Route* | Anesthetic Duration | Comments |
| Dissociative Combinations | |||
| Ketamine + midazolam | 10-15 mg/kg ketamine + 0.2-0.5 mg/kg midazolam IM |
Up to 30 minutes | Light anesthetic plane Minimal analgesia - frequently used in combination with an opioid Useful for non-invasive procedures Midazolam reversed with flumazenil |
| Ketamine + dexmedetomidine | 10-15 mg/kg ketamine + 0.01-0.02 mg/kg dexmedetomidine IM |
Up to 60 minutes | Light to moderate anesthetic plane Cardiorespiratory depression; oxygen supplementation is recommended Dexmedetomidine reversed with atipamezole |
| Others | |||
| Alfaxalone | 10-12 mg/kg SC | 60-90 minutes | Large volume Mild to moderate sedation when used alone Oxygen supplementation is recommended |
| Propofol | 1-4 mg/kg IV slow bolus 0.2-0.8 mg/kg/min IV constant rate infusion (CRI) |
5-7 minutes | Titrate to effect for anesthetic induction or used as a CRI as part of partial intravenous anesthesia (PIVA) Cardiorespiratory depression; oxygen supplementation is recommended Apnea is common |
*Intramuscular (IM), intravenous (IV), subcutaneous (SC). These can be used with opioids to produce analgesia with profound sedation or general anesthesia as below.
Reversal Agents Used in Ferrets
| Drug | Dosage/Route* | Reversal Category | Reversal for: |
| Atipamezole | 0.05-0.2 mg/kg SC, IM | Alpha-2 adrenergic receptors | Dexmedetomidine |
| Flumazenil | 0.02-0.08 mg/kg SC, IM, IV | Benzodiazepines | Midazolam |
| Naloxone | 0.1-1 mg/kg SC, IM, IV | Opioids | Buprenorphine, hydromorphone, etc. |
*Subcutaneous (SC), intramuscular (IM), intravenous (IV)
Analgesics
- Examples of common analgesic agents are listed below. Others are available; please contact RARC veterinarians to discuss alternatives such as gabapentin, tramadol, etc.
- Although the below agents are used, studies on analgesic efficacy are sparse in ferrets.
- Unrelieved pain has profound physiologic consequences, which may alter research results.
- Pain assessment in ferrets consists of evaluating behavioral and physiologic parameters and use of pain scoring systems such as the Ferret Grimace Scale along with other clinical signs (Reijgwart et al. 2017; van Zeeland and Shoemaker 2023).
- Ferret pain behaviors include decreased activity, decreased appetite, inactivity, staying curled or reluctance to curl, stiff gait, muscle fasciculations, fluffed tail, aggression, tooth grinding, and vocalization. The pain score and other signs must be documented in the record along with any analgesic intervention taken.
- The IACUC requires the use of preemptive analgesia (analgesics given prior to the first skin incision) for all survival surgical procedures unless scientifically justified.
- Pre-emptive analgesics may decrease the amount of required anesthetic drugs; doses of both injectable and inhalant agents should be adjusted accordingly.
Analgesic Agents Used in Ferrets
| Drug | Dosage/Route* | Frequency | Comments |
| Opioids/Sodium Channel Blockers/NMDA Antagonists | |||
| Buprenorphine (300 mcg/mL) | 0.03-0.06 mg/kg IM, IV, TM | 4-6 hours | Partial mu-opioid agonist Mild to moderate analgesia Slow onset |
| Extended-release buprenorphine (Ethiqa XR) | 0.6 mg/kg SC | Up to 72 hours | Partial mu-opioid agonist Mild to moderate analgesia Slow onset |
| Hydromorphone | 0.1-0.2 mg/kg IM, IV, SC | 1-4 hours | Full mu opioid agonist Profound analgesia |
| Butorphanol | 0.5-0.7 mg/kg IM, IV | 1-2 hours | Mu opioid antagonist; kappa opioid agonist Mild, short analgesia |
| Fentanyl | 2-5 mcg/kg bolus IV 2-20 mcg/kg/hr IV |
Continuous rate infusion (CRI) | Full mu agonist Profound analgesia |
| Ketamine | 0.25-1 mg/kg bolus SC, IV 2-20 mcg/kg/min IV |
CRI | Excellent somatic analgesia Reduces inhalant required |
| NSAIDs | |||
| Meloxicam | 0.2 mg/kg SC, PO | 12-24 hours | COX-2 selective Can titrate down to 0.05 mg/kg PO every other day for chronic use |
| Local Anesthetics** | |||
| Liposomal encapsulated bupivacaine (Nocita®) | Up to 0.4 mL/kg intra-incisional infiltration | Up to 3 days | Must cover entire incision due to limited diffusion Favorable safety profile |
| Lidocaine | Up to 6 mg/kg SC | 1-2 hours | Usually not recommended due to short duration of action |
| Bupivacaine | Up to 2 mg/kg SC | 6-8 hours | Peak onset ~10 minutes |
*Subcutaneous (SC), intraperitoneal (IP), intravenous (IV), oral (PO), transmucosal (TM). **Note: lidocaine and bupivacaine/Nocita® should not be mixed together.
Inhalant Anesthetics
Inhalant anesthetic delivery methods:
- Mask or chamber induction is not used due to personnel exposure, slow induction times and inability to control the airway.
- Anesthetic maintenance using a mask can be performed but is not recommended due to personnel exposure, inability to protect the airway from regurgitation, and inability to control ventilation.
- Endotracheal intubation is recommended during anesthetic maintenance; RARC personnel should be contacted for more information concerning endotracheal intubation in ferrets.
- A carrier gas is required; usually it is ~100% oxygen.
- Common settings:
- Immediately following induction: ~2 L/min; maintenance: ~ 180 mL/kg/min.
- Common settings:
- Isoflurane and sevoflurane can both be used in ferrets. However, they require separate vaporizers due to different vapor pressures and the MAC values (see Overview of Anesthetic and Analgesia Procedures tab).
- Common vaporizer settings:
- Isoflurane: immediately following induction ~2-4%, maintenance ~1-2%
- Sevoflurane: immediately following induction ~4-6%, maintenance ~3-4%
- Common vaporizer settings:
- Inhalants are profound cardiovascular and respiratory depressants; premedication is required to reduce amount of inhalant required.
- Inhalants require proper breathing and scavenging systems; RARC personnel can assist in choosing appropriate systems for your laboratory.
- For ferrets, a non-rebreathing system must be used to reduce resistance to breathing. Minimum oxygen flow rates are ~ 180 mL/kg/min to prevent rebreathing.
Endotracheal Intubation
- See Overview of Anesthetic and Analgesia Procedures tab for additional information. Please consult with RARC personnel if you are performing tracheal intubation in ferrets.
- Adult ferret endotracheal tubes are usually 2.5-4.0 mm; kits require much smaller tubes and, depending on age/size, can be a small as 1.5 mm.
- Laryngoscopes are used to ensure proper visualization of the larynx for atraumatic intubation. Fiberoptic laryngoscope blades should be used.
- The mouth should be opened with ties behind the interdigitated canine teeth. The laryngoscope blade should be placed on the base of the tongue – not on the epiglottis – to visualize the trachea. 2% lidocaine (~ 0.05 mL in an adult) is placed on the arytenoid cartilages. The endotracheal tube cuff should be lubricated with a sterile, water-soluble lubricant to form an appropriate seal. The endotracheal tube is gently placed curved side down through the glottis and the cuff inflated only to an airway pressure of ~ 20 cm H2O and no higher using the minimal occlusion pressure technique.
- Local anesthetics with benzocaine may induce methemoglobinemia – DO NOT USE!
- DO NOT simply put air in the cuff – ONLY inflate it to seal at 20 cm H2
- Gentle techniques must be used; trauma is common and laryngeal/tracheal rupture can occur. Pneumomediastinum and pneumothorax may result from overzealous intubation attempts.
- Please contact RARC if you have questions concerning this procedure.
Anesthetic Monitoring
Anesthetic monitoring techniques:
- Although constant vigilance is required during anesthesia (ferrets should never be unattended), record vital signs every 5-10 to immediately see values and to assess changes over time. RARC can provide you with an anesthetic record template if needed.
- Vital signs
- Cardiovascular system vital signs:
- Heart rate/pulse rate
- Can be taken using a pulse oximeter (see below), palpation of the heart or stethoscope.
- Normal ~180-250 beats/minute, depending on anesthetic agents used.
- Arterial blood pressures
- Measured using oscillometric devices or by placement of direct lines into an artery.
- Mean arterial pressure should always be kept above ~ 70 mmHg to ensure organ perfusion.
- Heart rate/pulse rate
- Respiratory system vital signs:
- Respiratory rate
- This can be measured by visualizing the movement of the animal’s chest wall as it rises and falls or via the end-tidal carbon dioxide monitor (capnometer).
- Normal respiratory rates typically are ~35-40 breaths/min.
- Capnography
- Veterinary-specific monitors measure end-tidal CO2 levels and produce capnograph waves. These are useful in detecting abnormalities in ventilation and appropriate tracheal intubation.
- Values will read artificially low when a non-rebreathing circuit is used.
- Pulse oximetry
- Veterinary-specific monitors are available to measure the amount of oxygen saturation of hemoglobin in blood (normal = >95%) and pulse rate.
- Readings could be impaired by reduced pulsatile strength (hypotension, hypothermia, vasoconstriction, etc.).
- Care must be taken if probe is placed on tongue due to potential sloughing from high clamp pressures.
- Respiratory rate
- Anesthetic depth
- Jaw tone, palpebral reflexes and limb withdrawal reflexes are performed to determine appropriate anesthetic depth prior to a procedure.
- Body temperature
- Ferrets can quickly become hypothermic or hyperthermic during anesthesia (normal = 98.6-104 °F).
- Rectal or esophageal thermometers or implanted telemeters should be used.
- Temperature should not decrease lower than ~ 98°F throughout the procedure; circulating warm water blankets, forced warm air circulators and warmed anesthetic circuits should be used.
- Surgical clipping and scrubbing or prepping agents should be minimized; ferrets should not be “soaked” with solutions.
- Electrical blankets, rice bags, water bottles, and gloves are NOT allowed because they frequently produce burns.
- Pain scoring
- Pain scoring systems such as the Ferret Grimace Scale along with other clinical signs (Reijgwart et al. 2017; van Zeeland and Shoemaker 2023) must be used and documented in the record.
- Analgesics must be administered as deemed necessary by intervention levels and per protocol or in discussion with RARC veterinary personnel.
- Pain scoring systems such as the Ferret Grimace Scale along with other clinical signs (Reijgwart et al. 2017; van Zeeland and Shoemaker 2023) must be used and documented in the record.
- Cardiovascular system vital signs:
Fluid Therapy
- Intra-operative anesthetic fluid rate should be ~5-7 mL/kg/hr IV using balanced crystalloid solutions (Plasmalyte, Normosol, Lactated Ringer’s, etc.). Fluid rates and types should be adjusted based on individual animal needs.
- Voluntary fluid intake should be documented after the procedure into the recovery period.
- Once fully recovered, supplemental fluid can be given IV or SC if required. Supplemental fluids should consist of a balanced crystalloid (Plasmalyte, Normosol, Lactated Ringer’s, etc.) and may be supplemented with other substances such as glucose (not SC).
Recovery
Factors that contribute to a successful recovery:
- Environment
- Recovery observations must be documented every 5-10 minutes until fully ambulating; a template can be provided by RARC if required.
- Recovery areas should be warm and quiet with dim, yet sufficient lighting to appropriately observe the ferrets.
- If appropriate room temperatures cannot be achieved, the incorporation of supplemental heat sources should be used as above.
- Ferrets should be extubated at the first sign of tongue movement or return of the swallow to reduce laryngospasm. Ferrets clench their interdigitating teeth tightly and must be extubated before the tube is bitten. The ability to maintain sternal recumbency must be ensured.
- Proper hydration and GI function
- Supplemental fluid support (as described above), nutritional support (moist food, enriched food, etc.) and/or active feeding techniques may be necessary and beneficial. Palatable food should be offered when ferrets are ambulating and have fully regained their swallow reflex to reduce GI stasis.
- Like fluid intake, food intake should be documented in the record and monitored regularly.
Additional Resources
Consult the RARC Veterinary Staff (vet@rarc.wisc.edu) if you have specific questions about the anesthetic procedures included in your IACUC protocol.
For assistance with anesthesia equipment set-up, please contact the RARC Trainers (trainer@rarc.wisc.edu).
Form templates to record anesthesia monitoring are also available on the RARC website.
RARC loans out select anesthesia equipment to the UW-Madison research community for temporary use. Please visit the RARC website to find out what items are available to borrow.
Useful Text Resources:
Veterinary Anesthetic and Monitoring Equipment by Cooley and Johnson
UW-Madison Libraries provides free access to the online version of this textbook.
Veterinary Anesthesia and Analgesia: The Sixth Edition of Lumb and Jones by Lamont et al.
UW-Madison Libraries provides free access to the online version of this textbook.
Exotic Animal Formulary by Carpenter et al.
UW-Madison Libraries provides free access to the online version of this textbook.
Works Cited
“Anesthesia (Guideline).” Anesthesia (Guideline)|Vertebrate Animal Research, https://animal.research.uiowa.edu/iacuc-guidelines-anesthesia.
Danneman PJ and Fish RE. Anesthesia and Analgesia in Laboratory Animals (Second Edition). Academic Press, 2008.
Flecknell PA. Laboratory Animal Anaesthesia Ed. 4. Academic Press, 2015.
Lamont L et al. Veterinary Anesthesia and Analgesia: The Sixth Edition of Lumb and Jones. Wiley Blackwell, 2024.
Reijgwart, Marsinah L et al. “The composition and initial evaluation of a grimace scale in ferrets after surgical implantation of a telemetry probe.” PloS one vol. 12,11 e0187986. 13 Nov. 2017, doi:10.1371/journal.pone.0187986
“Supplemental Anesthesia Training Resource.” Received by Rebecca Johnson, Supplemental Anesthesia Training Resource, 7 May 2020.
van Zeeland, Yvonne, and Nico Schoemaker. “Pain Recognition in Ferrets.” The veterinary clinics of North America. Exotic animal practice vol. 26,1 (2023): 229-243. doi:10.1016/j.cvex.2022.07.011
RARC’s “Overview of Anesthetic and Analgesic Procedures” must be read prior to accessing the below information.
Contact your RARC veterinarian prior to choosing an anesthetic, including agents that are NOT listed below.
Species-Specific Considerations
Pre-Anesthetic Considerations
- An acclimation period of at least 3 days is required prior to any procedure.
- Body weights must be obtained for appropriate drug dosing.
- Gerbils are curious and playful. They groom, wrestle, purr, thump, burrow, and gnaw frequently.
- Fasting and water restriction is not routinely performed in gerbils due to high metabolic rates and inability to vomit.
- Despite anesthetic chosen, sterile ophthalmic ointment should be applied to eyes since blink reflexes are significantly reduced during anesthesia and sedation.
- It is very important to consult your RARC veterinarian when determining what anesthetic is best for your study.
- Neonatal gerbils require unique considerations; please consult your RARC veterinarian for specific recommendations.
Recommended Agents
Injectable Sedatives, Anesthetics, and Analgesics
Sedatives/Anesthetics
- Examples of sedative and anesthetic agents are included below, along with reversal agents.
- Detailed information concerning agents can be found at: Lamont et al, The Sixth Edition of Lumb & Jones.
- For many researchers, ketamine combinations are the preferred choice of injectable anesthesia methods in gerbils, although inhalant techniques are recommended.
- Classified as a dissociative anesthetic and an NMDA receptor antagonist; DEA schedule III-controlled substance.
- Ketamine alone produces muscle rigidity; therefore, it is commonly paired with other agents to produce a more comprehensive anesthetic experience for the animal.
- Ketamine can be diluted if necessary for accurate dosing.
- If an opioid is used as a premedication, lower doses of other agents are used.
- Injectable agents NOT recommended: alpha-chloralose, chloral hydrate, tribromoethanol.
Injectable Anesthetics Used in Gerbils
| Drug | Dosage/Route* | Anesthetic Duration | Comments |
| Dissociative Combinations | |||
| Ketamine + dexmedetomidine |
75 mg/kg ketamine + 0.25 mg/kg dexmedetomidine IP |
30-60 minutes sedation/anesthesia | Sedation to anesthesia, depending on dose Re-dose with 1/3 of ketamine dose only or use supplemental inhalant (~0.5-1.0%) for additional anesthesia Cardiorespiratory depression; oxygen supplementation is recommended Dexmedetomidine is reversed with atipamezole |
| Barbiturates | |||
| Pentobarbital | 50-90 mg/kg IP | 10-300 minutes | Cardiorespiratory depression and poor analgesia Narrow safety margin Only appropriate prior to terminal perfusions/euthanasia Not recommended for survival surgery |
| Others | |||
| Urethane | 1-2 g/kg IP, IV | 360-480 minutes | Minimal cardiorespiratory depression Carcinogenic - requires proper precautions and SOP for handling Terminal procedures only unless scientific justification provided |
*Intraperitoneal (IP), intravenous (IV)
Reversal Agents Used in Gerbils
| Drug | Dosage/Route | Reversal Category | Reversal for: |
| Atipamezole | 1 mg/kg SC, IP | Alpha-2 adrenergic receptors | Dexmedetomidine |
| Naloxone | 0.1-1 mg/kg SC, IP, IV | Opioids | Buprenorphine, morphine, etc. |
*Subcutaneous (SC), intraperitoneal (IP), intravenous (IV)
Analgesics
- Examples of common analgesic agents are listed below. Others are available; please contact RARC veterinarians to discuss alternatives.
- Unrelieved pain has profound physiologic consequences, which may alter research results.
- Pain assessment in gerbils is challenging. However, procedures that are presumed to be painful warrant the provision of analgesia, despite the lack of indicators of pain.
- Although inconsistent, signs of pain in some gerbils include hunched posture, piloerection, reluctance to move, depression or aggression, weight loss, licking the incision, decreased overall grooming, twitching, arching the back, vocalization, etc.
- Information regarding effective analgesics in gerbils is limited.
- The IACUC requires the use of preemptive analgesia (analgesics given prior to the first skin incision) for all survival surgical procedures unless scientifically justified.
- Pre-emptive analgesics may decrease the amount of required anesthetic drugs; doses of both injectable and inhalant agents should be adjusted accordingly.
Analgesic Agents Used in Gerbils
| Drug | Dosage/Route* | Frequency | Comments |
| Opioids | |||
| Buprenorphine (300 mcg/mL) | 0.1-0.5 mg/kg IP, SC | 6-8 hours | Partial mu-opioid agonist |
| Extended-release buprenorphine (Ethiqa-XR®) | 1 mg/kg SC | Up to 72 hours | FDA indexed Partial mu-opioid agonist Pica is common |
| NSAIDs | |||
| Carprofen | 5 mg/kg SC | 24 hours | COX-2 selective |
| Meloxicam | 1-2 mg/kg SC | 24 hours | COX-2 selective |
| Ketoprofen | 5 mg/kg SC | 24 hours | Non-selective NSAID |
| Local Anesthetics ** Doses must be calculated carefully due to toxicity potential with overdose (cardiac, CNS, etc.) |
|||
| Liposomal encapsulated bupivacaine (Nocita®) | Up to 0.4 mL/kg intra-incisional infiltration | Up to 3 days | Must cover entire incision due to limited diffusion Favorable safety profile |
| Lidocaine | Up to 6 mg/kg SC | 1-2 hours | Dilution of parent compound is frequently required Usually not recommended due to short duration of action |
| Bupivacaine | Up to 2 mg/kg SC | 4-6 hours | Dilution of parent compound may be required Peak onset ~10 minutes |
*Subcutaneous (SC), intraperitoneal (IP), intravenous (IV), oral (PO).
**Note: lidocaine and bupivacaine/Nocita® should not be mixed together.
Inhalant Anesthetics
Inhalant anesthetic delivery methods:
- Induction chambers, along with nosecones and face masks, are commonly used in gerbils for induction and maintenance of general anesthesia.
- A carrier gas is required; usually it is ~100% oxygen. Low flow systems are available.
- Common settings:
- Induction: ~0.5-1 L/min; maintenance: ~0.5-1 L/min for a standard, single non-rebreathing system. Different systems may have other carrier gas flow requirements – please check with manufacturers or the RARC trainers.
- Common settings:
- Isoflurane and sevoflurane can both be used in gerbils. However, they require separate vaporizers due to different vapor pressures and the MAC values differ (see Overview of Anesthetic and Analgesia Procedures tab).
- Common vaporizer settings:
- Isoflurane: induction ~2-4%, maintenance ~1-2%
- Sevoflurane: induction ~4-6%, maintenance ~3-4%
- Common vaporizer settings:
- Inhalants are profound cardiovascular and respiratory depressants; premedication is required to smooth induction/recovery and reduce amount of inhalant required.
- Both require proper delivery equipment and a scavenging system; RARC personnel can assist in choosing appropriate systems for your laboratory.
- Can be delivered either via an agent-specific vaporizer or drop box.
- Chamber induction
- Controlled delivery of a specific inhalant concentration via a vaporizer; excess gas is scavenged.
- Inhalant should be turned off before chamber lid is opened to reduce personnel exposure or entire procedure should be performed in a ducted fume hood or biosafety cabinet.
- Drop jar/Drop box method
- Not a recommended method due to lack of anesthetic concentration control and potential exposure of personnel to high inhalant concentrations.
- Used for brief and single uses only. Should not be used for any surgical or prolonged procedures.
- Gas must be scavenged. Procedure must be performed under ducted fume hood or biosafety cabinet.
- Gerbils must not contact liquid anesthetic, which is placed on a cotton ball, gauze, etc.
- The drop box must be cleaned after every animal to limit contamination.
- Chamber induction
“Anesthesia (Guidelines) / Vertebrate Animal Research”- University of Iowa
| Isoflurane Concentration in Drop Box | 1L | 2L | 3L | 4L | 5L |
| 1% | 0.05mL | 0.10 mL | 0.15 mL | 0.20 mL | 0.26 mL |
| 2% | 0.10 mL | 0.20 mL | 0.31 mL | 0.41 mL | 0.51 mL |
| 3% | 0.15 mL | 0.31 mL | 0.46 mL | 0.61 mL | 0.77 mL |
| 4% | 0.20 mL | 0.41 mL | 0.61 mL | 0.82 mL | 1.02 mL |
| 5% | 0.26 mL | 0.51 mL | 0.77 mL | 1.02 mL | 1.28 mL |
Endotracheal Intubation
- See Overview of Anesthetic and Analgesia Procedures tab for additional information.
- Endotracheal intubation of gerbils is difficult - please contact RARC if you have questions concerning this procedure.
Anesthetic Monitoring
Anesthetic monitoring techniques:
- Although constant vigilance is required during anesthesia (gerbils should never be unattended), record vital signs every 5-10 minutes to immediately see values and to assess changes over time. RARC can provide you with an anesthetic record template if needed.
- Vital signs
- For smaller laboratory animals (e.g., gerbils), required vital sign monitoring may be adapted from the signs discussed below:
- Cardiovascular system vital signs:
- Heart rate/pulse rate
- Can be taken using a pulse oximeter (see below), palpation of the heart or stethoscope.
- Normal ~300-600 beats/minute, depending on anesthetic agents used.
- Arterial blood pressures
- Measured using rodent-specific oscillometric devices or by placement of direct lines into an artery.
- Heart rate/pulse rate
- Respiratory system vital signs:
- Respiratory rate
- This can be measured by visualizing the movement of the animal’s chest wall as it rises and falls or via the end-tidal carbon dioxide monitor (capnometer).
- Normal respiratory rates are species dependent but typically are ~80-120 breaths/min in gerbils; respiratory rate significantly drops under anesthesia in gerbils.
- Capnography
- Rodent-specific monitors are available to measure end-tidal CO2 levels in gerbils.
- Pulse oximetry
- Rodent-specific monitors are available to measure the amount of oxygen saturation of hemoglobin in blood (normal = >95%) and pulse rate in gerbils.
- Readings could be impaired by reduced pulsatile strength (hypotension, hypothermia, vasoconstriction, etc.).
- Respiratory rate
- Anesthetic depth
- Palpebral reflexes and limb withdrawal reflexes are performed to determine appropriate anesthetic depth prior to a procedure.
- Body temperature
- Gerbils can quickly become hypothermic during anesthesia but may be become hyperthermic as well (normal = 98.6-100.4 °F).
- Rectal thermometers created for rodents or implanted telemeters must be used.
- Temperature must not decrease lower than ~98°F throughout the procedure; circulating warm water blankets, forced warm air circulators and warmed anesthetic circuits should be used.
- Excess surgical clipping/scrubbing and excessive drenching of gerbils with prepping agents must be minimized.
- Electrical blankets, rice bags, water bottles, and gloves are NOT allowed because they frequently produce burns.
- Pain scoring
- Although signs of pain are inconsistent in gerbils, any change in behavior or other physiologic parameters must be documented in the record and veterinary staff notified.
- Administer analgesia as deemed necessary by intervention levels and per protocol or in discussion with the veterinary staff.
Fluid Therapy
- Intra-operative anesthetic fluid rate should be ~10 mL/kg/hr SC or IP using balanced crystalloid solutions (Plasmalyte, Normosol, Lactated Ringer’s, etc.). Fluid rates and types should be adjusted based on individual animal needs.
- Voluntary fluid intake must be documented after the procedure into the recovery period.
- Once fully recovered, supplemental fluids consisting of a balanced crystalloid (Plasmalyte, Normosol, Lactated Ringer’s, etc.) can be given SC or IP. Oral glucose and electrolyte-containing gels may also be used.
Recovery
Factors that contribute to a successful recovery:
- Environment
- Recovery observations must be documented every 5-10 minutes until fully ambulating; a template can be provided by RARC if required.
- Gerbils must not be moved to their normal housing cage until fully recovered. Premature transport to their home cage can result in the rodent inhaling bedding (such as corn cob), leading to airway obstruction or pneumonia. Instead, they should be transferred to a cage that has no bedding or a thin, paper layer. Heat should be applied as needed (see above) underneath the recovery cage. By placing the supplemental heat source under half of the recovery cage, it gives the animal the option and ability to self-regulate their temperature as they regain consciousness.
- Proper hydration and GI function
- Reduced fecal production can be due to pain, lack of fecal material in the GI tract or ileus. If ileus is suspected, contact RARC veterinary personnel.
- Supplemental fluid support (as described above), nutritional support (moist food, enriched gels, etc.) and/or feeding using oral gavage may be necessary and beneficial.
- Like fluid intake, food intake must be documented and monitored regularly.
Additional Resources
Consult the RARC Veterinary Staff (vet@rarc.wisc.edu) if you have specific questions about the anesthetic procedures included in your IACUC protocol.
For assistance with anesthesia equipment set-up, please contact the RARC Trainers (trainer@rarc.wisc.edu).
Form templates to record anesthesia monitoring are also available on the RARC website.
RARC loans out select anesthesia equipment to the UW-Madison research community for temporary use. Please visit the RARC website to find out what items are available to borrow.
Useful Text Resources:
Veterinary Anesthetic and Monitoring Equipment by Cooley and Johnson
UW-Madison Libraries provides free access to the online version of this textbook.
Veterinary Anesthesia and Analgesia: The Sixth Edition of Lumb and Jones by Lamont et al.
UW-Madison Libraries provides free access to the online version of this textbook.
Exotic Animal Formulary by Carpenter et al.
UW-Madison Libraries provides free access to the online version of this textbook.
Works Cited
“Anesthesia (Guideline).” Anesthesia (Guideline)|Vertebrate Animal Research, https://animal.research.uiowa.edu/iacuc-guidelines-anesthesia.
Danneman PJ and Fish RE. Anesthesia and Analgesia in Laboratory Animals (Second Edition). Academic Press, 2008.
Flecknell PA. Laboratory Animal Anaesthesia Ed. 4. Academic Press, 2015.
Lamont L et al. Veterinary Anesthesia and Analgesia: The Sixth Edition of Lumb and Jones. Wiley Blackwell, 2024.
“Supplemental Anesthesia Training Resource.” Received by Rebecca Johnson, Supplemental Anesthesia Training Resource, 7 May 2020.
RARC’s “Overview of Anesthetic and Analgesic Procedures” must be read prior to accessing the below information.
Contact your RARC veterinarian prior to choosing an anesthetic, including agents that are NOT listed below.
Species-Specific Considerations
Pre-Anesthetic Considerations
- An acclimation period of at least 3 days is required prior to any procedure.
- Body weights must be obtained for appropriate drug dosing.
- Fasting for 2 hours is recommended in guinea pigs to empty their stomach and cecum except in very young or compromised animals. Water is never withheld.
- Guinea pigs store feed in their cheek pouches, and this can lead to airway obstruction. To reduce the amount of material stored in the cheek pouches, the mouth should be gently rinsed with tap water (10-20 mL) and the inside of both cheeks gently swabbed with a cotton tipped applicator to remove any remaining material prior to anesthesia.
- SC and IM injections are common. The preferred IM injection site is within the lumbar epaxial musculature or the quadriceps muscle. The biceps femoris is not recommended due to the possibility of injecting near the sciatic nerve.
- An IV catheter should always be placed to administer additional anesthetic agents, emergency drugs, and/or fluids. The cephalic or lateral saphenous veins are the preferred site; lidocaine-prilocaine (EMLA) cream can be placed 30-60 minutes prior to reduce pain with catheterization.
- Despite anesthetic chosen, sterile ophthalmic ointment should be applied to eyes since blink reflexes are significantly reduced during anesthesia and sedation.
- It is very important to consult your RARC veterinarian when determining what anesthetic is best for your study.
- Neonatal guinea pigs require unique considerations; please consult your RARC veterinarian for specific recommendations.
Recommended Agents
Injectable Sedatives, Anesthetics, and Analgesics
Sedatives/Anesthetics
- Examples of sedative and anesthetic agents are included below, along with reversal agents.
- Detailed information concerning agents can be found at: Lamont et al, The Sixth Edition of Lumb & Jones.
- For many researchers, ketamine combinations are the preferred choice of injectable sedative/anesthetic methods in guinea pigs, although inhalant anesthetic techniques are recommended.
- Classified as a dissociative anesthetic and an NMDA receptor antagonist; DEA schedule III-controlled substance.
- Ketamine alone produces muscle rigidity; therefore, it is commonly paired with other agents to produce a more comprehensive anesthetic experience for the animal.
- Ketamine can be diluted if necessary for accurate dosing.
- Popular ketamine combinations (+/- opioid) (see below)
- Ketamine/xylazine
- Ketamine/dexmedetomidine
- Ketamine/midazolam
- If an opioid is used as a premedication, lower doses of other agents are used.
Sedatives Used in Guinea Pigs
| Drug | Dosage/Route* | Duration | Comments |
| Midazolam | 1-2 mg/kg SC, IM, IV | 30-45 minutes | Mild to moderate sedation Reversed with flumazenil Frequently combined with other agents (alfaxalone, ketamine) for moderate sedation/light anesthesia |
| Alpha-2 agonists: Dexmedetomidine OR Xylazine |
0.025-0.030 mg/kg SC, IM |
30-45 minutes | Mild to profound sedation, based on dose Some analgesia Reversed with atipamezole |
| Alfaxalone | 5 mg/kg IM | ~30 minutes | Moderate sedation Apnea common Frequently combined with other agents for improved muscle relaxation/sedation |
*Subcutaneous (SC), Intramuscular (IM), intravenous (IV)
Injectable Anesthetics Used in Guinea Pigs
| Drug | Dosage/Route& | Anesthetic Duration | Comments |
| Dissociative Combinations/Propofol | |||
| Ketamine + xylazine |
20-50 mg/kg ketamine + 3-5 mg/kg xylazine IM, IV |
60-90 minutes sedation/anesthesia | Sedation to anesthesia, depending on dose Cardiorespiratory depression; oxygen supplementation is recommended Re-dose with 1/3 ketamine dose only or use inhalant for additional anesthesia Xylazine is reversed with atipamezole |
| Ketamine + dexmedetomidine |
20-40 mg/kg ketamine + 0.025-0.03 mg/kg dexmedetomidine IM, IV |
30-60 minutes sedation/anesthesia | Sedation to anesthesia, depending on dose Cardiorespiratory depression; oxygen supplementation is recommended Re-dose with 1/3 ketamine dose only or use inhalant for additional anesthesia Dexmedetomidine is reversed with atipamezole |
| Ketamine + midazolam |
20-30 mg/kg ketamine + 1-2 mg/kg midazolam IM, IV |
30-45 minutes | Deep sedation/mild anesthesia Not appropriate as a sole surgical agent Midazolam is reversed with flumazenil |
| Propofol | 3-10 mg/kg IV slow bolus | 5-7 minutes | Titrate to effect for anesthetic induction Cardiorespiratory depression; oxygen supplementation is recommended Apnea is common |
| Barbiturates | |||
| Pentobarbital | 15-30 mg/kg IP | 10-300 minutes | Cardiorespiratory depression and poor analgesia Narrow safety margin Only appropriate prior to terminal perfusions/euthanasia Not recommended for survival surgery |
*Intramuscular (IM), intraperitoneal (IP), intravenous (IV)
Reversal Agents Used in Guinea Pigs
| Drug | Dosage/Route* | Reversal Category | Reversal for: |
| Atipamezole | 0.5-1 mg/kg SC, IM | Alpha-2 adrenergic receptors | Dexmedetomidine Xylazine |
| Flumazenil | 0.05-0.1 mg/kg SC, IP, IV | Benzodiazepines | Midazolam |
| Naloxone | 0.1-1 mg/kg SC, IM, IV | Opioids | Buprenorphine, morphine, etc. |
*Subcutaneous (SC), intramuscular (IM), intraperitoneal (IP), intravenous (IV)
Analgesics
- Examples of common analgesic agents are listed below. Others are available; please contact RARC veterinarians to discuss alternatives.
- Unrelieved pain has profound physiologic consequences, which may alter research results.
- Pain assessment in guinea pigs is challenging. However, procedures that are presumed to be painful warrant the provision of analgesia, despite the lack of indicators of pain.
- Signs of pain in guinea pigs include piloerection, reluctance to move, decreased appetite, vocalization, depression or lethargy, decreased grooming, abnormal posturing, weight loss, licking the painful area, grinding teeth, etc. Ethograms and pain scales have been used with minimal effectiveness (Oliver et al. 2017; Benedetti et al. 2024).
- Information regarding effective analgesics in guinea pigs is limited.
- The IACUC requires the use of preemptive analgesia (analgesics given prior to the first skin incision) for all survival surgical procedures unless scientifically justified.
- Pre-emptive analgesics may decrease the amount of required anesthetic drugs; doses of both injectable and inhalant agents should be adjusted accordingly.
Analgesic Agents Used in Guinea Pigs
| Drug | Dosage/Route* | Frequency | Comments |
| Opioids | |||
| Buprenorphine (300 mcg/mL) | 0.05 mg/kg IP, SC | 6-8 hours | Partial mu-opioid agonist |
| Extended-release buprenorphine (Ethiqa-XR®) | 0.48 mg/kg SC | Up to 72 hours | FDA indexed Partial mu-opioid agonist May take up to 8 hours to reach effective plasma levels |
| Hydromorphone | 0.1-0.2 mg/kg IM, IV | 4-6 hours | Full mu opioid agonist Profound analgesia |
| Methadone | 0.2-0.6 mg/kg IM, IV | 4-6 hours | Full mu opioid agonist and NMDA antagonist Profound analgesia |
| NSAIDS | |||
| Carprofen | 4 mg/kg SC | 24 hours | COX-2 selective |
| Meloxicam | 0.1-0.3 mg/kg SC, PO | 24 hours | COX-2 selective |
| Ketoprofen | 1 mg/kg SC | 12-24 hours | Non-selective NSAID |
| Local Anesthetics ** Doses must be calculated carefully due to toxicity potential with overdose (cardiac, CNS, etc.) |
|||
| Liposomal encapsulated bupivacaine (Nocita®) | Up to 0.4 mL/kg intra-incisional infiltration | Up to 3 days | Must cover entire incision due to limited diffusion Favorable safety profile |
| Lidocaine | Up to 6 mg/kg SC | 1-2 hours | Dilution of parent compound is frequently required Usually not recommended due to short duration of action |
| Bupivacaine | Up to 2 mg/kg SC* | 4-6 hours | Dilution of parent compound may be required Peak onset ~10 minutes |
*Subcutaneous (SC), intraperitoneal (IP), intravenous (IV), oral (PO).
**Note: lidocaine and bupivacaine/Nocita® should not be mixed together.
Inhalant Anesthetics
Inhalant anesthetic delivery methods:
- Sedation must occur prior to mask induction with inhalant agents; these procedures are not recommended due to personnel exposure, slow induction times and inability to control the airway.
- Agent-specific vaporizers are required, and excess gas must be scavenged.
- Tight-fitting face masks are used to deliver oxygen +/- inhalant anesthetics.
- Inhalant should be turned off before the mask is removed or the entire procedure performed in a ducted fume hood or biosafety cabinet.
- Anesthetic maintenance using a nosecone or mask can be used.
- A carrier gas is required; usually it is ~100% oxygen.
- Common settings:
- Induction: ~1-2 L/min; maintenance: ~0.5-1 L/min, depending on flow requirements of the system.
- Common settings:
- Isoflurane and sevoflurane can both be used in guinea pigs. However, they require separate vaporizers due to different vapor pressures and the MAC values (see Overview of Anesthetic and Analgesia Procedures tab).
- Common vaporizer settings:
- Isoflurane: induction ~2-4%, maintenance ~1-2 %
- Sevoflurane: induction ~4-6%, maintenance ~3-4%
- Common vaporizer settings:
- Inhalants are profound cardiovascular and respiratory depressants; premedication is required to smooth induction/recovery and reduce amount of inhalant required.
- Both require proper delivery equipment and a scavenging system; RARC personnel can assist in choosing appropriate systems for your laboratory.
Endotracheal Intubation
- See Overview of Anesthetic and Analgesia Procedures tab for additional information.
- Endotracheal intubation of guinea pigs is difficult - please contact RARC if you have questions concerning this procedure.
Anesthetic Monitoring
Anesthetic monitoring techniques:
- Although constant vigilance is required during anesthesia (guinea pigs should never be unattended), record vital signs every 5-10 minutes to immediately see values and to assess changes over time. RARC can provide you with an anesthetic record template if needed.
- Vital signs
- For smaller laboratory animals (e.g., guinea pigs), required vital sign monitoring may be adapted from the signs discussed below:
- Cardiovascular system vital signs:
- Heart rate/pulse rate
- Can be taken using a pulse oximeter (see below), palpation of the heart or stethoscope.
- Normal ~250-380 beats/minute, depending on anesthetic agents used. Heart rate may be slower if alpha-2 agonists are used.
- Arterial blood pressures
- Measured using rodent-specific oscillometric devices or by placement of direct lines into an artery.
- Heart rate/pulse rate
- Respiratory system vital signs:
- Respiratory rate
- This can be measured by visualizing the movement of the animal’s chest wall as it rises and falls or via the end-tidal carbon dioxide monitor (capnometer).
- Normal respiratory rates are species dependent but typically are ~50-100 breaths/min in guinea pigs; respiratory rate significantly drops under anesthesia.
- Capnography
- Rodent-specific monitors are available to measure end-tidal CO2 levels in guinea pigs with a tight-fitting mask.
- Pulse oximetry
- Rodent-specific monitors are available to measure the amount of oxygen saturation of hemoglobin in blood (normal = >95%) and pulse rate.
- Readings could be impaired by reduced pulsatile strength (hypotension, hypothermia, vasoconstriction, etc.).
- Respiratory rate
- Anesthetic depth
- Palpebral reflexes and limb withdrawal reflexes are performed to determine appropriate anesthetic depth prior to a procedure.
- Body temperature
- Guinea pigs can quickly become hypothermic during anesthesia but may become hyperthermic as well (normal = 99-103.1 °F).
- Rectal thermometers created for rodents or implanted telemeters must be used.
- Temperature must not decrease lower than ~98°F throughout the procedure; circulating warm water blankets, forced warm air circulators and warmed anesthetic circuits should be used.
- Excess surgical clipping/scrubbing and excessive drenching of guinea pigs with prepping agents must be minimized.
- Electrical blankets, rice bags, water bottles, and gloves are NOT allowed because they frequently produce burns.
- Pain scoring
- Any change in behavior or other physiologic parameters associated with pain must be documented in the record and veterinary staff notified.
- Analgesics should be administered as deemed necessary by intervention levels and per protocol or in discussion with the veterinary staff.
Fluid Therapy
- Intra-operative anesthetic fluid rate should be ~10 mL/kg/hr SC or IV using balanced crystalloid solutions (Plasmalyte, Normosol, Lactated Ringer’s, etc.). Fluid rates and types should be adjusted based on individual animal needs.
- Voluntary fluid intake must be documented after the procedure into the recovery period.
- Once fully recovered, supplemental fluids consisting of a balanced crystalloid (Plasmalyte, Normosol, Lactated Ringer’s, etc.) can be given SC or IV. Oral glucose and electrolyte-containing gels may also be used.
Recovery
Factors that contribute to a successful recovery:
- Environment
- Recovery observations must be documented every 5-10 minutes until fully ambulating; a template can be provided by RARC if required.
- Guinea pigs must not be moved to their normal housing cage until fully recovered. Premature transport to their home cage can result in the rodent inhaling bedding (such as corn cob), leading to airway obstruction or pneumonia. Instead, they should be transferred to a cage that has no bedding or a thin, paper layer. Heat should be applied as needed (see above) underneath the recovery cage. By placing the supplemental heat source under half of the recovery cage, it gives the animal the option and ability to self-regulate their temperature as they regain consciousness.
- Proper hydration and GI function
- Reduced fecal production can be due to pain, lack of fecal material in the GI tract or ileus. If ileus is suspected, RARC veterinary personnel should be contacted.
- Supplemental fluid support (as described above), nutritional support (moist food, enriched gels, etc.) and/or feeding using oral gavage may be necessary and beneficial.
- Like fluid intake, food intake must be documented and monitored regularly.
Additional Resources
Consult the RARC Veterinary Staff (vet@rarc.wisc.edu) if you have specific questions about the anesthetic procedures included in your IACUC protocol.
For assistance with anesthesia equipment set-up, please contact the RARC Trainers (trainer@rarc.wisc.edu).
Form templates to record anesthesia monitoring are also available on the RARC website.
RARC loans out select anesthesia equipment to the UW-Madison research community for temporary use. Please visit the RARC website to find out what items are available to borrow.
Useful Text Resources:
Veterinary Anesthetic and Monitoring Equipment by Cooley and Johnson
UW-Madison Libraries provides free access to the online version of this textbook.
Veterinary Anesthesia and Analgesia: The Sixth Edition of Lumb and Jones by Lamont et al.
UW-Madison Libraries provides free access to the online version of this textbook.
Exotic Animal Formulary by Carpenter et al.
UW-Madison Libraries provides free access to the online version of this textbook.
Works Cited
“Anesthesia (Guideline).” Anesthesia (Guideline)|Vertebrate Animal Research, https://animal.research.uiowa.edu/iacuc-guidelines-anesthesia.
Danneman PJ and Fish RE. Anesthesia and Analgesia in Laboratory Animals (Second Edition). Academic Press, 2008.
Flecknell PA. Laboratory Animal Anaesthesia Ed. 4. Academic Press, 2015.
Lamont L et al. Veterinary Anesthesia and Analgesia: The Sixth Edition of Lumb and Jones. Wiley Blackwell, 2024.
“Supplemental Anesthesia Training Resource.” Received by Rebecca Johnson, Supplemental Anesthesia Training Resource, 7 May 2020.
RARC’s “Overview of Anesthetic and Analgesic Procedures” must be read prior to accessing the below information.
Contact your RARC veterinarian prior to choosing an anesthetic, including agents that are NOT listed below.
Species-Specific Considerations
Pre-Anesthetic Considerations
- An acclimation period of at least 3 days is required prior to any procedure.
- Body weights must be obtained for appropriate drug dosing.
- Fasting and water restriction is not routinely performed in hamsters due to high metabolic rates and inability to vomit.
- Hamsters store feed in their cheek pouches, and this can lead to airway obstruction. To reduce the amount of material stored in the cheeks, the mouth should be gently rinsed with tap water (10-20 mL) and the inside of both cheeks gently swabbed with a cotton tipped applicator to remove any remaining material prior to anesthesia.
- Despite anesthetic chosen, sterile ophthalmic ointment should be applied to eyes since blink reflexes are significantly reduced during anesthesia and sedation.
- It is very important to consult your RARC veterinarian when determining what anesthetic is best for your study.
- Neonatal hamsters require unique considerations; please consult your RARC veterinarian for specific recommendations.
Recommended Agents
Injectable Sedatives, Anesthetics, and Analgesics
Sedatives/Anesthetics
- Examples of sedative and anesthetic agents are included below, along with reversal agents.
- Detailed information concerning agents can be found at: Lamont et al, The Sixth Edition of Lumb & Jones.
- For many researchers, ketamine combinations are the preferred choice of injectable anesthesia methods in hamsters.
- Classified as a dissociative anesthetic and an NMDA receptor antagonist; DEA schedule III-controlled substance.
- Ketamine alone produces muscle rigidity; therefore, it is commonly paired with other agents to produce a more comprehensive anesthetic experience for the animal.
- Ketamine can be diluted if necessary for accurate dosing.
- Popular ketamine combinations (+/- opioid):
- Ketamine/xylazine
- Ketamine/dexmedetomidine
- If an opioid is used as a premedication, lower doses of other agents are used
- Injectable agents NOT recommended: alpha-chloralose, chloral hydrate, tribromoethanol.
Injectable Anesthetics Used in Hamsters
| Drug | Dosage/Route& | Anesthetic Duration | Comments |
| Dissociative Combinations | |||
| Ketamine + xylazine |
100-200 mg/kg ketamine + 5-7 mg/kg xylazine IP |
30-60 minutes sedation/anesthesia | Sedation to anesthesia, depending on dose Re-dose with 1/3 of ketamine dose only or use supplemental inhalant (~0.5-1.0%) for additional anesthesia Cardiorespiratory depression; oxygen supplementation is recommended Xylazine is reversed with atipamezole |
| Ketamine + dexmedetomidine |
75-100 mg/kg ketamine + 0.25 mg/kg dexmedetomidine IP |
30-60 minutes sedation/anesthesia | Re-dose with 1/3 of ketamine dose only or use supplemental inhalant (~0.5-1.0%) for additional anesthesia Cardiorespiratory depression; oxygen supplementation is recommended Dexmedetomidine is reversed with atipamezole |
| Barbiturates | |||
| Pentobarbital | 50-90 mg/kg IP | 10-300 minutes | Cardiorespiratory depression and poor analgesia Narrow safety margin Only appropriate prior to terminal perfusions/euthanasia Not recommended for survival surgery |
| Others | |||
| Urethane | 1-2 g/kg IP, IV | 360-480 minutes | Minimal cardiorespiratory depression Carcinogenic - requires proper precautions and SOP for handling Terminal procedures only unless scientific justification provided |
*Intraperitoneal (IP), intravenous (IV)
Reversal Agents Used in Hamsters
| Drug | Dosage/Route* | Reversal Category | Reversal for: |
| Atipamezole | 1 mg/kg SC, IP | Alpha-2 adrenergic receptors | Dexmedetomidine Xylazine |
| Naloxone | 0.1-1 mg/kg SC, IP, IV | Opioids | Buprenorphine, morphine, etc. |
*Subcutaneous (SC), intraperitoneal (IP), intravenous (IV)
Analgesics
- Examples of common analgesic agents are listed below. Others are available; please contact RARC veterinarians to discuss alternatives.
- Unrelieved pain has profound physiologic consequences, which may alter research results.
- Pain assessment in hamsters is challenging. However, procedures that are presumed to be painful warrant the provision of analgesia, despite the lack of indicators of pain.
- Although inconsistent, signs of pain in some hamsters include hunched posture, piloerection, reluctance to move, depression or aggression, weight loss, licking the incision, decreased overall grooming, twitching, arching the back, vocalization, etc. In addition, the Syrian Hamster Grimace Scale and Treat Take Test have been used but remain inconsistent (Edmunson et al. 2021).
- Information regarding effective analgesics in hamsters is limited.
- The IACUC requires the use of preemptive analgesia (analgesics given prior to the first skin incision) for all survival surgical procedures unless scientifically justified.
- Pre-emptive analgesics may decrease the amount of required anesthetic drugs; doses of both injectable and inhalant agents should be adjusted accordingly.
Analgesic Agents Used in Hamsters
| Drug | Dosage/Route* | Frequency | Comments |
| Opioids | |||
| Buprenorphine (300 mcg/mL) | 0.1-0.5 mg/kg IP, SC | 6-8 hours | Partial mu-opioid agonist |
| Extended-release buprenorphine (Ethiqa-XR®) | 0.8 mg/kg SC | Up to 72 hours | FDA indexed Partial mu-opioid agonist Pica is common |
| NSAIDs | |||
| Carprofen | 5 mg/kg SC | 24 hours | COX-2 selective |
| Meloxicam | 1-2 mg/kg SC | 24 hours | COX-2 selective |
| Ketoprofen | 5 mg/kg SC | 24 hours | Non-selective NSAID |
| Local Anesthetics** Doses must be calculated carefully due to toxicity potential with overdose (cardiac, CNS, etc.) |
|||
| Liposomal encapsulated bupivacane (Nocita®) | Up to 0.4 mL/kg intra-incisional infiltration | Up to 3 days | Must cover entire inicision due to limited diffusion Favorable safety profile |
| Lidocaine | Up to 6 mg/kg SC | 1-2 hours | Dilution of parent compound is frequently required Usually not recommended due to short duration of action |
| Bupivacaine | Up to 2 mg/kg SC | 4-6 hours | Dilution of parent compound may be required Peak onset ~10 minutes |
*Subcutaneous (SC), intraperitoneal (IP), intravenous (IV), oral (PO).
**Note: lidocaine and bupivacaine/Nocita® should not be mixed together.
Inhalant Anesthetics
-
Inhalant anesthetic delivery methods:
- Induction chambers, along with nosecones and face masks are commonly used in hamsters for induction and maintenance of general anesthesia.
- A carrier gas is required; usually it is ~100% oxygen. Low flow systems are available.
- Common settings:
- Induction: ~0.5-1 L/min; maintenance: ~0.5-1 L/min for a standard, single non-rebreathing system. Different systems may have other carrier gas flow requirements – please check with manufacturers or the RARC trainers.
- Isoflurane and sevoflurane can both be used in hamsters. However, they require separate vaporizers due to different vapor pressures and the MAC values differ (see Overview of Anesthetic and Analgesia Procedures tab).
- Common vaporizer settings:
- Isoflurane: induction ~2-4%, maintenance ~1-2%
- Sevoflurane: induction ~4-6%, maintenance ~3-4%
- Inhalants are profound cardiovascular and respiratory depressants; premedication is required to smooth induction/recovery and reduce amount of inhalant required.
- Both require proper delivery equipment and a scavenging system; RARC personnel can assist in choosing appropriate systems for your laboratory.
- Can be delivered either via an agent-specific vaporizer or drop box.
- Chamber induction
- Controlled delivery of a specific inhalant concentration via a vaporizer; excess gas is scavenged.
- Inhalant should be turned off before chamber lid is opened to reduce personnel exposure or entire procedure should be performed in a ducted fume hood or biosafety cabinet.
- Drop jar/Drop box method
- Not a recommended method due to lack of anesthetic concentration control and potential exposure of personnel to high inhalant concentrations.
- Used for brief and single uses only. Should not be used for any surgical or prolonged procedures.
- Gas must be scavenged. Procedure must be performed under ducted fume hood or biosafety cabinet.
- Hamsters must not contact liquid anesthetic, which is placed on a cotton ball, gauze, etc.
- The drop box must be cleaned after every animal to limit contamination.
- Chamber induction
- Common vaporizer settings:
- Common settings:
“Anesthesia (Guidelines)/ Vertebrate Animal Research”- University of Iowa
Isoflurane Concentration in Drop Box
1L
2L
3L
4L
5L
1%
0.05mL
0.10 mL
0.15 mL
0.20 mL
0.26 mL
2%
0.10 mL
0.20 mL
0.31 mL
0.41 mL
0.51 mL
3%
0.15 mL
0.31 mL
0.46 mL
0.61 mL
0.77 mL
4%
0.20 mL
0.41 mL
0.61 mL
0.82 mL
1.02 mL
5%
0.26 mL
0.51 mL
0.77 mL
1.02 mL
1.28 mL
Endotracheal Intubation
- See Overview of Anesthetic and Analgesia Procedures tab for additional information.
- Endotracheal intubation of hamsters is difficult - please contact RARC if you have questions concerning this procedure.
Anesthetic Monitoring
Anesthetic monitoring techniques:
- Although constant vigilance is required during anesthesia (hamsters should never be unattended), record vital signs every 5-10 minutes to immediately see values and to assess changes over time. RARC can provide you with an anesthetic record template if needed.
- Vital signs
- For smaller laboratory animals (e.g., hamsters), required vital sign monitoring may be adapted from the signs discussed below:
- Cardiovascular system vital signs:
- Heart rate/pulse rate
- Can be taken using a pulse oximeter (see below), palpation of the heart or stethoscope.
- Normal ~300-600 beats/minute, depending on anesthetic agents used.
- Arterial blood pressures
- Measured using rodent-specific oscillometric devices or by placement of direct lines into an artery.
- Heart rate/pulse rate
- Respiratory system vital signs:
- Respiratory rate
- This can be measured by visualizing the movement of the animal’s chest wall as it rises and falls or via the end-tidal carbon dioxide monitor (capnometer).
- Normal respiratory rates are species dependent but typically are ~80-120 breaths/min in hamsters; respiratory rate significantly drops under anesthesia in hamsters.
- Capnography
- Rodent-specific monitors are available to measure end-tidal CO2 levels in hamsters as well.
- Pulse oximetry
- Rodent-specific monitors are available to measure the amount of oxygen saturation of hemoglobin in blood (normal = >95%) and pulse rate in hamsters as well.
- Readings could be impaired by reduced pulsatile strength (hypotension, hypothermia, vasoconstriction, etc.).
- Respiratory rate
- Anesthetic depth
- Palpebral reflexes and limb withdrawal reflexes are performed to determine appropriate anesthetic depth prior to a procedure.
- Body temperature
- Hamsters can quickly become hypothermic during anesthesia but may become hyperthermic as well (normal = 98.6-100.4 °F).
- Rectal thermometers created for rodents or implanted telemeters must be used.
- Temperature must not decrease lower than ~98°F throughout the procedure; circulating warm water blankets, forced warm air circulators and warmed anesthetic circuits should be used.
- Excess surgical clipping/scrubbing and excessive drenching of hamsters with prepping agents must be minimized.
- Electrical blankets, rice bags, water bottles, and gloves are NOT allowed because they frequently produce burns.
- Pain scoring
- Although sign of pain are inconsistent in hamsters, any change in behavior or other physiologic parameters must be documented in the record and veterinary staff notified.
- Analgesics should be administered as deemed necessary by intervention levels and per protocol or in discussion with the veterinary staff.
Fluid Therapy
- Intra-operative anesthetic fluid rate should be ~10 mL/kg/hr SC or IP using balanced crystalloid solutions (Plasmalyte, Normosol, Lactated Ringer’s, etc.). Fluid rates and types should be adjusted based on individual animal needs.
- Voluntary fluid intake must be documented after the procedure into the recovery period.
- Once fully recovered, supplemental fluids consisting of a balanced crystalloid (Plasmalyte, Normosol, Lactated Ringer’s, etc.) can be given SC or IP. Oral glucose and electrolyte-containing gels may also be used.
Recovery
Factors that contribute to a successful recovery:
- Environment
- Recovery observations must be documented every 5-10 minutes until fully ambulating; a template can be provided by RARC if required.
- Hamsters must not be moved to their normal housing cage until fully recovered. Premature transport to their home cage can result in the rodent inhaling bedding (such as corn cob), leading to airway obstruction or pneumonia. Instead, they should be transferred to a cage that has no bedding or a thin, paper layer. Heat should be applied as needed (see above) underneath the recovery cage. By placing the supplemental heat source under half of the recovery cage, it gives the animal the option and ability to self-regulate their temperature as they regain consciousness.
- Proper hydration and GI function
- Reduced fecal production can be due to pain, lack of fecal material in the GI tract or ileus. If ileus is suspected, RARC veterinary personnel should be contacted.
- Supplemental fluid support (as described above), nutritional support (moist food, enriched gels, etc.) and/or feeding using oral gavage may be necessary and beneficial.
- Like fluid intake, food intake must be documented and monitored regularly.
Additional Resources
Consult the RARC Veterinary Staff (vet@rarc.wisc.edu) if you have specific questions about the anesthetic procedures included in your IACUC protocol.
For assistance with anesthesia equipment set-up, please contact the RARC Trainers (trainer@rarc.wisc.edu).
Form templates to record anesthesia monitoring are also available on the RARC website.
RARC loans out select anesthesia equipment to the UW-Madison research community for temporary use. Please visit the RARC website to find out what items are available to borrow.
Useful Text Resources:
Veterinary Anesthetic and Monitoring Equipment by Cooley and Johnson
UW-Madison Libraries provides free access to the online version of this textbook.
Veterinary Anesthesia and Analgesia: The Sixth Edition of Lumb and Jones by Lamont et al.
UW-Madison Libraries provides free access to the online version of this textbook.
Exotic Animal Formulary by Carpenter et al.
UW-Madison Libraries provides free access to the online version of this textbook.
Works Cited
“Anesthesia (Guideline).” Anesthesia (Guideline)|Vertebrate Animal Research, https://animal.research.uiowa.edu/iacuc-guidelines-anesthesia.
Danneman PJ and Fish RE. Anesthesia and Analgesia in Laboratory Animals (Second Edition). Academic Press, 2008.
Flecknell PA. Laboratory Animal Anaesthesia Ed. 4. Academic Press, 2015.
Lamont L et al. Veterinary Anesthesia and Analgesia: The Sixth Edition of Lumb and Jones. Wiley Blackwell, 2024.
“Supplemental Anesthesia Training Resource.” Received by Rebecca Johnson, Supplemental Anesthesia Training Resource, 7 May 2020.
RARC’s “Overview of Anesthetic and Analgesic Procedures” must be read prior to accessing the below information.
Contact your RARC veterinarian prior to choosing an anesthetic, including agents that are NOT listed below.
Species-Specific Considerations
Pre-Anesthetic Considerations
- An acclimation period of at least 3 days is required prior to any procedure.
- Body weights must be obtained for appropriate drug dosing.
- Fasting and water restriction is not routinely performed in mice due to high metabolic rates and inability to vomit.
- Despite anesthetic chosen, sterile ophthalmic ointment should be applied to the eyes since blink reflexes are significantly reduced during anesthesia and sedation.
- It is very important to consult your RARC veterinarian when determining what anesthetic is best for your study.
- Neonatal mice require unique considerations; please consult your RARC veterinarian for specific recommendations.
Recommended Agents
Injectable Sedatives, Anesthetics, and Analgesics
Sedatives/Anesthetics
- Examples of sedative and anesthetic agents are included below, along with reversal agents.
- Detailed information concerning agents can be found at: Lamont et al, The Sixth Edition of Lumb & Jones.
- For many researchers, ketamine combinations are the preferred choice of injectable anesthesia methods in mice.
- Classified as a dissociative anesthetic and NMDA receptor antagonist; DEA schedule III-controlled substance.
- Ketamine alone produces muscle rigidity; therefore, it is commonly paired with other agents to produce a more comprehensive anesthetic experience for the animal.
- Popular ketamine combinations (+/- opioid):
- Ketamine/xylazine
- Ketamine/dexmedetomidine
- If an opioid is used as a premedication, lower doses of other agents are used.
- Injectable agents NOT recommended: alpha-chloralose, chloral hydrate, tribromoethanol.
Sedatives Used in Mice
| Drug | Dosage/Route* | Duration | Comments |
| Dissociative Combinations | |||
| Ketamine + midazolam |
100 mg/kg ketamine + 5 mg/kg midazolam IP |
20-30 minutes | Sedation only |
| Ketamine + acepromazine |
100 mg/kg ketamine + 5 mg/kg acepromazine IP |
20-30 minutes | Sedation only |
| Others | |||
| Alfaxalone | Females: 40-80 mg/kg IP Males: 80-120 mg/kg IP |
30-60 minutes | Sedation only No analgesia Apnea can be seen |
* Intraperitoneal (IP)
Injectable Anesthetics Used in Mice
| Drug | Dosage/Route* | Anesthetic Duration | Comments |
| Dissociative Combinations | |||
| Ketamine + xylazine |
75-150 mg/kg ketamine + 5-10 mg/kg xyalzine IP |
30-45 minutes surgical anesthesia | Re-dose with 1/2 of ketamine dose only or use supplemental inhalant (~0.5-1.0%) for additional anesthesia Cardiorespiratory depression; oxygen supplementation is recommended Xylazine is reversed with atipamezole |
| Ketamine + dexmedetomidine |
50-75 mg/kg ketamine + 0.5-1.0 mg/kg dexmedetomidine IP |
20-30 minutes surgical anesthesia | Re-dose with 1/2 of ketamine dose only or use supplemental inhalant (~0.5-1.0%) for additional anesthesia Cardiorespiratory depression; oxygen supplementation is recommended Dexmedetomidine is reversed with atipamezole |
| Ketamine + xylazine + acepromazine |
80-100 mg/kg ketamine + 5-10 mg/kg xylazine + 1-2 mg/kg acepromazine IP |
40 minutes surgical anesthesia | Re-dose with 1/2 of ketamine dose, or 1/4 of ketamine dose and 1/4 xylazine dose Cardiorespiratory depression; oxygen supplementation is recommended Xylazine is reversed with atipamezole |
| Barbiturates | |||
| Pentobarbital | 30-60 mg/kg IP | 10-300 minutes | Cardiorespiratory depression and poor analgesia Narrow safety margin Only appropriate prior to terminal perfusions/euthanasia Not recommended for survival surgery |
| Others | |||
| Alfaxalone | Females: 40-80 mg/kg IP Males: 80-120 mg/kg IP |
30-60 minutes | Requires xylazine or dexmedetomidine or other agent for surgical anesthesia Cardiorespiratory depression; oxygen supplementation is recommended Apnea is common |
| Urethane | 1.3-1.8 g/kg IP, IV | 360-480 minutes | Minimal cardiorespiratory depression Carcinogenic - requires proper precautions and SOP for handling Terminal procedures only unless scientific justification provided |
*Intraperitoneal (IP), intravenous (IV)
Reversal Agents Used in Mice
| Drug | Dosage/Route* | Reversal Category | Reversal for: |
| Atipamezole | 0.5-1 mg/kg SC, IP | Alpha-2 adrenergic receptors | Dexmedetomidine Xylazine |
| Flumazenil | 0.1-10 mg/kg SC, IP, IV | Benzodiazepines | Midazolam |
| Naloxone | 0.1-1 mg/kg SC, IP, IV | Opioids | Buprenorphine, morphine, etc. |
*Subcutaneous (SC), intraperitoneal (IP), intravenous (IV)
Analgesics
- Examples of common analgesic agents are listed below. Others are available; please contact RARC veterinarians to discuss alternatives.
- Unrelieved pain has profound physiologic consequences, which may alter research results.
- Pain assessment in mice consists of evaluating behavioral and physiologic parameters and use of the Grimace Scale (see Analgesia Standard Treatment Guidelines for Laboratory Mice).
- In addition to facial signs of pain (Grimace Scale), behavioral signs of pain include: reluctance to move, hunched posture, social isolation, decreased appetite, decreased grooming, decreased nest building, increased aggression, self-aggression, and nasal/ocular porphyrin staining and should be documented in the record along with the Grimace Scale and any analgesic intervention taken.
- The IACUC requires the use of preemptive analgesia (analgesics given prior to the first skin incision) for all survival surgical procedures unless scientifically justified.
- Preemptive analgesics may decrease the amount of required anesthetic drugs; doses of both injectable and inhalant agents should be adjusted accordingly.
Analgesic Agents Used in Mice
| Drug | Dosage/Route* | Frequency | Comments |
| Opioids | |||
| Buprenorphine (300 mcg/mL) | 0.1-0.5 mg/kg IP, SC | 4-6 hours | Partial mu-opioid agonist Pica is common |
| Extended-release buprenorphine (Ethiqa-XR®) | 3.25 mg/kg SC | Up to 72 hours | FDA indexed Partial mu-opioid agonist Pica is common |
| Compounded extended-release buprenorphine (Buprenorphine Base-ER Lab) | 0.6 mg/kg SC | Up to 48 hours | Compounded Associated with skin nodules Partial mu-opioid agonist Pica is common Less recommended |
| NSAIDs | |||
| Carprofen | 5 mg/kg SC 20 mg/kg SC |
12 hours 24 hours |
COX-2 selective |
| Meloxicam | 5-10 mg/kg SC | 8-12 hours | COX-2 selective |
| Ketoprofen | 20 mg/kg SC | 24 hours | Non-selective NSAID |
| Local Anesthetics** Doses must be calculated carefully due to toxicity potential with overdose (cardiac, CNS, etc.) |
|||
| Liposomal encapsulated bupivacaine (Nocita®)** | Up to 0.4 mL/kg intra-incisional infiltration | Up to 3 days | Must cover entire incision due to limited diffusion Favorable safety profile |
| Lidocaine** | Up to 6 mg/kg SC | 1-2 hours | Dilution of parent compound is frequently required Usually not recommended due to short duration of action |
| Bupivacaine** | Up to 2 mg/kg SC | 6-8 hours | Dilution of parent compound may be required Peak onset ~ 10 minutes |
*Subcutaneous (SC), intraperitoneal (IP), intravenous (IV), oral (PO).
**Note: lidocaine and bupivacaine/Nocita® should not be mixed together.
Inhalant Anesthetics
Inhalant anesthetic delivery methods:
- Induction chambers, along with nosecones and face masks are commonly used in mice for induction and maintenance of general anesthesia.
- Endotracheal intubation is also used during anesthetic maintenance.
- A carrier gas is required; usually it is ~100% oxygen. Low flow systems are available.
- Common settings:
- Induction: ~0.5-1 L/min; maintenance: ~0.5-1 L/min for a standard, single non-rebreathing system. Different systems may have other carrier gas flow requirements – please check with manufacturers or the RARC trainers.
- Isoflurane and sevoflurane can both be used in mice. However, they require separate vaporizers due to different vapor pressures and the MAC values differ (see Overview of Anesthetic and Analgesia Procedures tab).
- Common vaporizer settings:
- Isoflurane: induction ~2-4%, maintenance ~1-2%
- Sevoflurane: induction ~4-6%, maintenance ~3-4%
- Inhalants are profound cardiovascular and respiratory depressants; premedication is required to smooth induction/recovery and reduce amount of inhalant required.
- Both require proper delivery equipment and a scavenging system; RARC personnel can assist in choosing appropriate systems for your laboratory.
- Can be delivered either via an agent-specific vaporizer or drop box.
- Chamber induction
- Controlled delivery of a specific inhalant concentration via a vaporizer; excess gas is scavenged.
- Inhalant should be turned off before chamber lid is opened to reduce personnel exposure or entire procedure should be performed in a ducted fume hood or biosafety cabinet.
- Drop jar/Drop box method
- Not a recommended method due to lack of anesthetic concentration control and potential exposure of personnel to high inhalant concentrations.
- Used for brief and single uses only. Should not be used for any surgical or prolonged procedures.
- Gas must be scavenged. Procedure must be performed under ducted fume hood or biosafety cabinet.
- Mice must contact liquid anesthetic, which is placed on a cotton ball, gauze, etc.
- The drop box must be cleaned after every animal to limit contamination.
- Chamber induction
- Common vaporizer settings:
- Common settings:
“Anesthesia (Guidelines) / Vertebrate Animal Research”- University of Iowa
| Isoflurane Concentration in Drop Box | 1L | 2L | 3L | 4L | 5L |
| 1% | 0.05mL | 0.10 mL | 0.15 mL | 0.20 mL | 0.26 mL |
| 2% | 0.10 mL | 0.20 mL | 0.31 mL | 0.41 mL | 0.51 mL |
| 3% | 0.15 mL | 0.31 mL | 0.46 mL | 0.61 mL | 0.77 mL |
| 4% | 0.20 mL | 0.41 mL | 0.61 mL | 0.82 mL | 1.02 mL |
| 5% | 0.26 mL | 0.51 mL | 0.77 mL | 1.02 mL | 1.28 mL |
Endotracheal Intubation
- See "Overview of Anesthetic and Analgesia Procedures" tab above for additional information.
- Rodent endotracheal tubes are usually custom-made out of intravenous (IV) catheters and select cannulas. Intubation can be achieved by placing the rodent in dorsal recumbency or by using commercially available, specialized-sloped restraining devices.
- Please contact RARC if you have questions concerning this procedure.
Anesthetic Monitoring
Anesthetic monitoring techniques:
- Although constant vigilance is required during anesthesia (mice should never be unattended), recording vital signs every 5-10 minutes is recommended to immediately see values and to assess changes over time. RARC can provide you with an anesthetic record template if needed.
- Vital signs
- For smaller laboratory animals (e.g., rodents), required vital sign monitoring may be adapted from the signs discussed below:
- Cardiovascular system vital signs:
- Heart rate/pulse rate
- Can be taken using a pulse oximeter (see below), palpation of the heart or stethoscope.
- Normal ~300-500 beats/minute, depending on anesthetic agents used.
- Arterial blood pressures
- Measured using rodent-specific oscillometric devices or by placement of direct lines into an artery.
- Respiratory system vital signs:
- Respiratory rate
- This can be measured by visualizing the movement of the animal’s chest wall as it rises and falls or via the end-tidal carbon dioxide monitor (capnometer).
- Normal respiratory rates are species dependent but typically are ~70-100 breaths/min in mice.
- Capnography
- Rodent-specific monitors are available to measure end-tidal CO2
- Pulse oximetry
- Rodent-specific monitors are available to measure the amount of oxygen saturation of hemoglobin in blood (normal = >95%) and pulse rate.
- Readings could be impaired by reduced pulsatile strength (hypotension, hypothermia, vasoconstriction, etc.).
- Anesthetic depth
- Palpebral reflexes and limb withdrawal reflexes are performed to determine appropriate anesthetic depth prior to a procedure.
- Body temperature
- Mice can quickly become hypothermic during anesthesia but may be become hyperthermic as well.
- Rectal thermometers specific to mice or implanted telemeters should be used.
- Temperature should not decrease lower than ~ 98°F throughout the procedure; circulating warm water blankets, forced warm air circulators and warmed anesthetic circuits should be used.
- Excess surgical clipping/scrubbing and excessive drenching of mice with prepping agents should be minimized.
- Electrical blankets, rice bags, water bottles, and gloves are NOT allowed because they frequently produce burns.
- Pain scoring
- Validated pain scoring systems such as the Grimace Scale should be used and documented on the Analgesia Standard Treatment Guidelines for Laboratory Mice tab.
- Analgesics should be administered as deemed necessary by intervention levels and per protocol or in discussion with the veterinary staff.
- Respiratory rate
- Heart rate/pulse rate
Fluid Therapy
- Intra-operative anesthetic fluid rate should be ~10 mL/kg/hr IV, SC, or IP using balanced crystalloid solutions (Plasmalyte, Normosol, Lactated Ringer’s, etc.). Fluid rates and types should be adjusted based on individual animal needs.
- Voluntary fluid intake should be documented after the procedure into the recovery period.
- Once fully recovered, supplemental fluids consisting of a balanced crystalloid (Plasmalyte, Normosol, Lactated Ringer’s, etc.) can be given SC or IP. Oral glucose and electrolyte-containing gels may also be used.
Recovery
Factors that contribute to a successful recovery:
- Environment
- Recovery observations should be documented every 5-10 minutes until fully ambulating; a template can be provided by RARC if required.
- Mice must not be moved to their normal housing cage until fully recovered. Premature transport to their home cage can result in the rodent inhaling bedding (such as corn cob), leading to airway obstruction or pneumonia. Instead, they should be transferred to a cage that has no bedding or a thin, paper layer. Heat should be applied as needed (see above) underneath the recovery cage. By placing the supplemental heat source under half of the recovery cage, it gives the animal the option and ability to self-regulate their temperature as they regain consciousness.
- Proper hydration and GI function
- Reduced fecal production can be due to pain, lack of fecal material in the GI tract or ileus. If ileus is suspected, RARC veterinary personnel should be contacted.
- Supplemental fluid support (as described above), nutritional support (moist food, enriched gels, etc.) and/or feeding using oral gavage may be necessary and beneficial.
- Like fluid intake, food intake should be documented and monitored regularly.
- If buprenorphine is administered, pica can be observed; digestible bedding material may be required to prevent GI obstruction.
Mouse Validated Pain Scoring System
Pain Scale: Mouse Grimace Scale
|
ITEM |
Description |
Score |
|
Orbital Tightening: - Narrowing of the orbital area, a tightly closed eyelid, or an eye squeeze - An eye closure that reduces the eye size by more than half should be coded as a “2” |
||
|
1 |
|
0 |
|
|
1 |
|
|
|
2 |
|
|
Nose Bulge: - The skin and muscles around the nose will be contracted, creating a rounded extension of skin visible on the bridge of the nose - Vertical wrinkles extending down the side of the nose from the bridge - In frontal headshots, a bulge may be seen as a widening of the nose area (i.e., V-shape connecting eyes to nose appears broader) |
||
|
2 |
|
0 |
|
|
1 |
|
|
|
2 |
|
|
Cheek Bulge: - The cheek muscle is contracted and extended relative to the baseline condition |
||
|
3 |
|
0 |
|
|
1 |
|
|
|
2 |
|
|
Ear Position: - The ears tend to rotate outwards and/or back, away from the face - The space between the ears may appear wider relative to baseline |
||
|
4 |
|
0 |
|
|
1 |
|
|
|
2 |
|
|
Whisker Changes: - Whiskers pulled back to lay flat against the cheek or pulled forward as if to be “standing on end” - Whiskers may also clump together |
||
|
5 |
|
0 |
|
|
1 |
|
|
|
2 |
|
Scale and Decision for Analgesic:
- Score total can range from 0 to 10
- Calculate the mean of the total score (total score/5)
- Analgesic intervention given at a score ≥ 4
Sedative, Anesthetic and Analgesic Dilutions for Mice
Store drugs in a cool, dry place shielded from light. Diluted drugs must be discarded 30 days after dilution.
Acepromazine (10 mg/mL): Dilute to 1 mg/mL
Draw 1 mL acepromazine (10 mg/mL) into a sterile syringe and dispense into a sterile multi-dose vial. Add 9 mL of sterile diluent (usually 0.9% NaCl). Label vial with “acepromazine 1 mg/mL”, initials of person who prepared it, the preparation date, and the expiration date.
Bupivacaine (5 mg/mL): Dilute to 2.5 mg/mL
Draw 1 mL bupivacaine (5 mg/mL) into a sterile syringe and dispense into a sterile multi-dose vial. Add 1 mL of sterile diluent (usually 0.9% NaCl). Label vial with “bupivacaine 2.5 mg/mL”, initials of person who prepared it, the preparation date, and the expiration date.
Buprenorphine HCl (0.3 mg/mL): Dilute to 0.005 mg/mL
Draw 0.1 mL buprenorphine (0.3 mg/mL) into a sterile syringe and dispense into a sterile multi-dose vial. Add 5.9 mL of sterile diluent (usually 0.9% NaCl). Label vial with “buprenorphine 0.005 mg/mL”, initials of person who prepared it, the preparation date, and the expiration date.
Carprofen (50 mg/mL): Dilute to 0.5 mg/mL
Draw 0.1 mL carprofen (50 mg/mL) into a sterile syringe and dispense into a sterile multi-dose vial. Add 9.9 mL of sterile diluent (usually 0.9% NaCl). Label vial with “carprofen 0.5 mg/mL”, initials of person who prepared it, the preparation date, and the expiration date.
Dexmedetomidine (0.5 mg/mL): Dilute to 0.25 mg/mL
Draw 1 mL of dexmedetomidine (0.5 mg/mL) into a sterile syringe and dispense into a sterile multi-dose vial. Add 1 mL of sterile diluent (usually 0.9% NaCl). Label vial with “dexmedetomidine 0.25 mg/mL”, initials of person who prepared it, the preparation date, and the expiration date.
Alternatively, purchase the more dilute, commercial preparation of dexmedetomidine (0.1 mg/mL).
Ketamine (100 mg/mL): Dilute to 50 mg/mL
Draw 1 mL of ketamine (100 mg/mL) into a sterile syringe and dispense into a sterile multi-dose vial. Add 1 mL of sterile diluent (usually 0.9% NaCl). Label vial with “ketamine 50 mg/mL”, initials of person who prepared it, the preparation date, and the expiration date.
Meloxicam (5 mg/mL): Dilute to 0.5 mg/mL
Draw 1 mL of meloxicam (5 mg/mL) into a sterile syringe and dispense into a sterile multi-dose vial. Add 9 mL of sterile diluent (usually 0.9% NaCl). Label vial with “meloxicam 0.5 mg/mL”, initials of person who prepared it, the preparation date, and the expiration date.
Midazolam (5 mg/mL): Dilute to 1 mg/mL
Draw 1 mL midazolam (5 mg/mL) into a sterile syringe and dispense into a sterile multi-dose vial. Add 4 mL of sterile diluent (usually 0.9% NaCl). Label vial with “midazolam 1 mg/mL”, initials of person who prepared it, the preparation date, and the expiration date.
Xylazine (20 mg/mL): Dilute to 2 mg/mL
Draw 1 mL xylazine (20 mg/mL) into a sterile syringe and dispense into a sterile multi-dose vial. Add 9 mL of sterile diluent (usually 0.9% NaCl). Label vial with “xylazine 2 mg/mL”, initials of person who prepared it, the preparation date, and the expiration date.
Additional Resources
Consult the RARC Veterinary Staff (vet@rarc.wisc.edu) if you have specific questions about the anesthetic procedures included in your IACUC protocol.
For assistance with anesthesia equipment set-up, please contact the RARC Trainers (trainer@rarc.wisc.edu).
Form templates to record anesthesia monitoring are available on the RARC website.
RARC loans out select anesthesia equipment to the UW-Madison research community for temporary use. Please visit the RARC website to find out what items are available to borrow.
Useful Text Resources:
Veterinary Anesthetic and Monitoring Equipment by Cooley and Johnson
UW-Madison Libraries provides free access to the online version of this textbook.
Veterinary Anesthesia and Analgesia: The Sixth Edition of Lumb and Jones by Lamont et al.
UW-Madison Libraries provides free access to the online version of this textbook.
Exotic Animal Formulary by Carpenter et al.
UW-Madison Libraries provides free access to the online version of this textbook.
Works Cited
“Anesthesia (Guideline).” Anesthesia (Guideline)|Vertebrate Animal Research, 10 May 2023. https://animal.research.uiowa.edu/iacuc-guidelines-anesthesia.
Danneman PJ and Fish RE. Anesthesia and Analgesia in Laboratory Animals (Second Edition). Academic Press, 2008.
Flecknell PA. Laboratory Animal Anaesthesia Ed. 4. Academic Press, 2015.
Lamont L et al. Veterinary Anesthesia and Analgesia: The Sixth Edition of Lumb and Jones. Wiley Blackwell, 2024.
“Supplemental Anesthesia Training Resource.” Received by Rebecca Johnson, Supplemental Anesthesia Training Resource, 7 May 2020.
RARC’s “Overview of Anesthetic and Analgesic Procedures” must be read prior to accessing the below information.
Contact your RARC veterinarian prior to choosing an anesthetic, including agents that are NOT listed below.
Species-Specific Considerations
Pre-Anesthetic Considerations
- An acclimation period of at least 3 days is required prior to any procedure.
- Body weights must be obtained for appropriate drug dosing.
- Personal protective equipment should always be worn when handling non-human primates to help prevent the transmission of zoonotic diseases (such as herpes B) to the handler or the spread of tuberculosis (TB) to the non-human primate (NHP).
- NHPs will resist restraint. Positive reinforcement training and acclimation to techniques should be utilized whenever possible to facilitate procedures without the use of chemical restraint or prior to anesthetic events.
- Smaller primates, such as marmosets, are typically easier to restrain when administering an IP or IM injection. Sedatives are administered prior to inhalant analgesia or if additional injectables are given IV. Large primates can injure their handler if they are not properly restrained. Therefore, use of pre-anesthetic drugs for chemical restraint administered IM are highly recommended.
- General anesthesia can be achieved by injecting IV via 20-24 gauge IV catheters in the cephalic, saphenous, or lateral tail vein, depending on primate species.
- Food should be withheld for ~ 6-12 hr to reduce regurgitation and/or vomiting.
- Maropitant (1 mg/kg) SC or slow IV may be administered to reduce vomiting.
- NHPs frequently have laryngospasm and stimulation of the caudal pharynx can stimulate vomiting. 2% lidocaine should be placed on the caudal larynx/pharynx prior to intubation attempts to reduce reflexes (see Tracheal Intubation).
- Despite anesthetic chosen, sterile ophthalmic ointment must be applied to eyes since blink reflexes are significantly reduced during anesthesia and sedation.
- It is very important to consult your RARC or WNPRC veterinarian when determining what anesthetic is best for your study.
- Neonatal and small NHPs (marmosets, tamarins, etc.) require unique considerations; please consult your RARC or WNPRC veterinarian for specific recommendations.
Recommended Agents
Injectable Sedatives, Anesthetics, and Analgesics
Sedatives/Anesthetics
- Examples of sedative and anesthetic agents are included below, along with reversal agents.
- Detailed information concerning agents can be found at: Lamont et al, The Sixth Edition of Lumb & Jones.
- Premedication is common and is administered IM prior to IV catheterization as discussed above.
- Premedication should be done in a squeeze cage and NHPs left in a quiet, dimmed area to achieve maximal sedation.
- Smaller NHP species generally require higher doses of agents than larger species based on metabolic scaling requirements; contact RARC or WNPRC veterinary personnel for specific doses required for your studies.
- Supplemental oxygen should always be administered throughout sedation and/or anesthesia.
Sedatives Used in NHPs
| Drug | Dosage/Route* | Duration | Comments |
| Acepromazine | 0.05-0.1 mg/kg IM, IV | 45-60 minutes | Mild sedation only Vasodilation and hypotension Usually used to prolong and smooth effects of other agents (ketamine) |
| Midazolam | 0.05-0.3 mg/kg IM, IV 0.05-0.3 mg/kg/hr IV constant rate infusion (CRI) |
30-60 minutes | Mild to moderate sedation Reversed with flumazenil Usually used to prolong and sooth effects of other agents (ketamine, alfaxalone) |
| Alpha-2 agonists: Dexmedetomidine |
0.001-0.03 mg/kg IM, IV 0.0005-0.001 mg/kg IV for emergence delirium |
30-60 minutes | Mild to profound sedation, based on dose Some analgesia Reversed with atipamezole |
*Intramuscular (IM), intravenous (IV). These can be used with other agents (such as opioids and dissociatives) to produce analgesia, profound sedation, or general anesthesia as below.
Injectable Anesthetics Used in NHPs
| Drug | Dosage/Route* | Anesthetic Duration | Comments |
| Dissociative Combinations | |||
| Ketamine + midazolam | 5-15 mg/kg ketamine + 0.05-0.3 mg/kg midazolam IM |
Up to 30 minutes | Immobilization Light anesthetic plane Minimal analgesia - frequently used in combination with an opioid Useful for non-invasive procedures Midazolam reversed with flumazenil |
| Ketamine + dexmedetomidine | 5-15 mg/kg ketamine + 0.01-0.03 mg/kg dexmedetomidine IM |
Up to 60 minutes | Light to moderate anesthetic plane Cardiorespiratory depression; oxygen supplementation is recommended Dexmedetomidine is reversed with atipamezole |
| Others | |||
| Alfaxalone | 0.5-5 mg/kg IM, IV | 10-30 minutes, depending on route and dose | Titrate to effect IV for anesthetic induction Large volume IM; combine with other agents to reduce volume Cardiorespiratory depression; oxygen supplementation is recommended Apnea is common |
| Propofol | 1-4 mg/kg IV slow bolus 0.2-0.8 mg/kg/min IV constant rate infusion (CRI) |
5-7 minutes | Titrate to effect for anesthetic induction or used as a CRI as part of partial intravenous anesthesia (PIVA) Cardiorespiratory depression; oxygen supplementation is recommended Apnea is common |
*Intramuscular (IM), intravenous (IV). These can be used with opioids to produce analgesia with profound sedation or general anesthesia as below.
NOTE: Ketamine administered alone has poor muscle relaxation. High doses increase intracranial and intraocular pressure. Salivation is common. It should be combined with other agents to reduce side effects and smooth the anesthetic procedure.
Reversal Agents Used in NHPs
| Drug | Dosage/Route* | Reversal Category | Reversal for: |
| Atipamezole | 0.05-0.2 mg/kg SC, IM | Alpha-2 adrenergic receptors | Dexmedetomidine |
| Flumazenil | 0.02-0.08 mg/kg SC, IM, IV | Benzodiazepines | Midazolam |
| Naloxone | 0.1-0.2 mg/kg SC, IM, IV | Opioids | Buprenorphine, hydromorphone, etc. |
*Subcutaneous (SC), intramuscular (IM), intravenous (IV)
Analgesics
- Examples of common analgesic agents are listed below. Others are available; please contact RARC or WNPRC veterinarians to discuss alternatives such as gabapentin, tramadol, etc.
- Unrelieved pain has profound physiologic consequences, which may alter research results.
- Pain assessment in NHPs consists of evaluating behavioral and physiologic parameters and use of validated pain scoring systems such as the Cynomolgus Macaque Grimace Scale (Paterson et al 2023).
- Pain scales may include orbital tightening, brow lowering, cheek tightening, hunched posture, abnormal gait, interactions with surroundings, reluctance to move, attention to the painful area, and appetite, among others. The pain score and other signs must be documented in the record along with any analgesic intervention taken.
- All mu and kappa opioid analgesics produce respiratory depression in NHPs; ventilation and oxygenation must be monitored (see Anesthetic Monitoring) when administered.
- The IACUC requires the use of preemptive analgesia (analgesics given prior to the first skin incision) for all survival surgical procedures unless scientifically justified.
- Preemptive analgesics may decrease the amount of required anesthetic drugs; doses of both injectable and inhalant agents should be adjusted accordingly.
Analgesic Agents Used in NHPs
| Drug | Dosage/Route* | Frequency | Comments |
| Opioid/Sodium Channel Blockers/NMDA Antagonists | |||
| Buprenorphine (300 mcg/mL) or high concentration buprenorphine (Simbadol™; 1.8 mg/mL) | 0.005-0.03 mg/kg IM, IV | 6-12 hours | Partial mu opioid agonist Mild to moderate analgesia Slow onset |
| Extended-release buprenorphine (Ethiqa XR) | 0.2 mg/kg SC | Up to 72 hours | Partial mu opioid agonist Mild to moderate analgesia 15 minute onset |
| Butorphanol | 0.1-0.2 mg/kg IM, IV | 2-3 hours | Kappa opioid agonist; mu opioid antagonist Milad analgesia Can reverse full mu opioid agonists while still providing mild analgesia |
| Hydromorphone | 0.05-0.1 mg/kg IM, IV | 4-6 hours | Full mu opioid agonist Moderate analgesia |
| Fentanyl | 2-5 mcg/kg bolus IV 2-10 mcg/kg/hr IV |
Constant Rate Infusion (CRI) | Full mu opioid agonist Profound analgesia |
| Lidocaine | 0.5-2 mg/kg bolus IV 50-75 mcg/kg/min IV |
Constant Rate Infusion (CRI) | Sodium channel blockade Anti-inflammatory, reduces inhalant required |
| Ketamine | 0.25-1 mg/kg bolus SC, IV 2-10 mcg/kg/min IV |
Constant Rate Infusion (CRI) | Excellent somatic analgesia Reduces inhalant required |
| NSAIDs and Antipyretics | |||
| Carprofen | 2-4 mg/kg SC, PO | 24 hours | COX-2 selective |
| Meloxicam | 0.2 mg/kg once followed by 0.1 mg/kg SC, PO | 24 hours | COX-2 selective |
| Acetaminophen | 5-10 mg/kg | 6 hours | NOT classified as an NSAID Centrally-acting analgesic May work at COX-3 pathway Antipyretic |
| Local Anesthetics | |||
| Liposomal encapsulated bupivacaine (Nocita®) | Up to 0.4 mL/kg intra-incisional infiltration | Up to 3 days | Must cover entire incision due to limited diffusion Favorable safety profile |
| Lidocaine | Up to 6 mg/kg SC | 1-2 hours | Usually not recommended due to short duration of action |
| Bupivacaine | Up to 2 mg/kg SC* | 6-8 hours | Peak onset ~10 minutes |
*Subcutaneous (SC), intraperitoneal (IP), intravenous (IV), oral (PO).
NOTE: lidocaine and bupivacaine/Nocita® should not be mixed together.
Inhalant Anesthetics
Inhalant anesthetic delivery methods:
- Mask induction is not recommended due to personnel exposure, slow induction times, and inability to control the airway, especially in species that vomit such as NHPs. If deemed necessary, sedation should precede mask induction and the diaphragm within the mask must result in a tight seal around the nose/mouth.
- Agent-specific vaporizers are required, and excess gas must be scavenged.
- Inhalant should be turned off before mask is removed.
- Anesthetic maintenance using a mask can be performed but is not recommended due to personnel exposure, inability to protect the airway from regurgitation, and inability to control ventilation.
- Endotracheal intubation is recommended during anesthetic maintenance; RARC personnel should be contacted for more information concerning endotracheal intubation in NHPs.
- A carrier gas is required; usually it is ~100% oxygen.
- Common settings:
- Immediately following induction: ~2 L/min; maintenance: ~1 L/min.
- If a non-rebreathing system is used in animals <3 kg, flow rates should be >180 mL/kg/min.
- Common settings:
- Isoflurane and sevoflurane can both be used in NHPs. However, they require separate vaporizers due to different vapor pressures and the MAC values (see Overview of Anesthetic and Analgesia Procedures tab).
- Common vaporizer settings:
- Isoflurane: immediately following induction ~2-4%, maintenance ~1-2%
- Sevoflurane: immediately following induction ~4-6%, maintenance ~3-4%
- Common vaporizer settings:
- Inhalants are profound cardiovascular and respiratory depressants; premedication is required to smooth induction/recovery and reduce amount of inhalant required.
- Inhalants require proper circle/rebreathing or non-rebreathing systems and scavenging systems; RARC personnel can assist in choosing appropriate systems for your laboratory.
Endotracheal Intubation
- See Overview of Anesthetic and Analgesia Procedures tab for additional information. Please consult with RARC personnel if you are performing tracheal intubation in NHPs.
- Orotracheal intubation can be difficult because of the angle between the oropharynx and tracheal opening, small oral cavity and incidence of laryngospasm.
- Larger NHP species frequently require 5-7 mm endotracheal tubes; neonates and smaller NHP species require much smaller tubes and depending on age/size can be a small as 2 mm.
- Endotracheal tube length must be measured beforehand since the trachea bifurcates prior to the thoracic inlet and to prevent one-lung (bronchial) intubation. Once intubated, bilateral pulmonary air flow must be auscultated with a stethoscope.
- Laryngoscopes are used to ensure proper visualization of the larynx for atraumatic intubation. Long fiberoptic laryngoscope blades should be used.
- NHPs can be intubated in dorsal or ventral recumbency with the neck flexed and mandible extended. The laryngoscope blade should be placed on the base of the tongue – not on the epiglottis – to visualize the trachea. If the rima glottidis cannot be seen, the soft palace should be displaced off of the epiglottis using the laryngoscope. 2% lidocaine (~ 0.1 mL in an adult) is placed on the arytenoid cartilages. The endotracheal tube cuff should be lubricated with a sterile, water-soluble lubricant to form an appropriate seal. The endotracheal tube is placed curved side down into the trachea to the pre-measured length to avoid bronchial intubation. It should be secured with an endotracheal tube tie.
- Gentle techniques must be used; trauma is common and laryngeal/tracheal rupture can occur.
- Please contact RARC if you have questions concerning this procedure.
Anesthetic Monitoring
Anesthetic monitoring techniques:
- Although constant vigilance is required during anesthesia (NHPs should never be unattended), record vital signs every 5-10 minutes to immediately see values and to assess changes over time. RARC can provide you with an anesthetic record template if needed.
- Vital signs
- Cardiovascular system vital signs:
- Heart rate/pulse rate
- Can be taken using a pulse oximeter (see below), palpation of the heart or stethoscope.
- Normal ~100-250 beats/minute, depending on species and anesthetic agents used.
- Arterial blood pressures
- Measured using oscillometric devices or by placement of direct lines into an artery.
- Mean arterial pressure should always be kept above ~ 70 mmHg to ensure organ perfusion.
- Respiratory system vital signs:
- Respiratory rate
- This can be measured by visualizing the movement of the animal’s chest wall as it rises and falls or via the end-tidal carbon dioxide monitor (capnometer).
- Normal respiratory rates typically are ~10-30 breaths/min, depending on species.
- Capnography
- Veterinary-specific monitors measure end-tidal CO2 levels and produce capnograph waves. These are useful in detecting abnormalities in ventilation and appropriate tracheal intubation.
- This is essential since NHPs experience significant respiratory depression under anesthesia.
- Pulse oximetry
- Veterinary-specific monitors are available to measure the amount of oxygen saturation of hemoglobin in blood (normal = >95%) and pulse rate.
- Readings could be impaired by reduced pulsatile strength (hypotension, hypothermia, vasoconstriction, etc.).
- Respiratory rate
- Anesthetic depth
- Jaw tone, palpebral reflexes and limb withdrawal reflexes are performed to determine appropriate anesthetic depth prior to a procedure.
- Body temperature
- NHPs can quickly become hypothermic or hyperthermic during anesthesia (normal = 97-102 °F, depending on species).
- Rectal or esophageal thermometers or implanted telemeters should be used.
- Temperature should not decrease lower than ~ 98°F throughout the procedure; circulating warm water blankets, forced warm air circulators and warmed anesthetic circuits should be used.
- Surgical clipping and scrubbing or prepping agents should be minimized.
- Electrical blankets, rice bags, water bottles, and gloves are NOT allowed because they frequently produce burns.
- Pain scoring
- Validated pain scoring systems such as the Cynomolgus Macaque Grimace Scale (Paterson et al 2023) must be used among other parameters and documented in the record.
- Analgesics should be administered as deemed necessary by intervention levels and per protocol or in discussion with RARC or WNPRC veterinary personnel.
- Heart rate/pulse rate
- Cardiovascular system vital signs:
Fluid Therapy
- Oral fluids supplemented with glucose may be administered pre- and post-procedure to help with peri-procedure hypoglycemia which is commonly encountered.
- Intra-operative anesthetic fluid rate should be ~5-10 mL/kg/hr IV using balanced crystalloid solutions (Plasmalyte, Normosol, Lactated Ringer’s, etc.). Fluid rates and types should be adjusted based on individual animal needs and species.
- Voluntary fluid intake should be documented after the procedure into the recovery period.
Recovery
Factors that contribute to a successful recovery:
- Environment
- Recovery observations must be documented every 5-10 minutes until fully ambulating; a template can be provided by RARC if required.
- Recovery areas should be warm and quiet with dim, yet sufficient lighting to appropriately observe the NHPs.
- If appropriate room temperatures cannot be achieved, the incorporation of supplemental heat sources should be used as appropriate.
- NHPs should be extubated at the first sign of the return of the laryngeal reflex. Appropriate restraint should be used and animals should be sitting upright in the recovery cage.
- Proper hydration and GI function
- Supplemental fluid support (as described above), nutritional support (moist food, enriched food, etc.) and/or active feeding techniques may be necessary and beneficial. Palatable food should be offered when NHPs are ambulating and have fully regained their swallow reflex to reduce GI stasis.
- Like fluid intake, food intake should be documented in the record and monitored regularly.
Additional Resources
Consult the RARC Veterinary Staff (vet@rarc.wisc.edu) if you have specific questions about the anesthetic procedures included in your IACUC protocol.
For assistance with anesthesia equipment set-up, please contact the RARC Trainers (trainer@rarc.wisc.edu).
Form templates to record anesthesia monitoring are also available on the RARC website.
RARC loans out select anesthesia equipment to the UW-Madison research community for temporary use. Please visit the RARC website to find out what items are available to borrow.
Useful Text Resources:
Veterinary Anesthetic and Monitoring Equipment by Cooley and Johnson
UW-Madison Libraries provides free access to the online version of this textbook.
Veterinary Anesthesia and Analgesia: The Sixth Edition of Lumb and Jones by Lamont et al.
UW-Madison Libraries provides free access to the online version of this textbook.
Exotic Animal Formulary by Carpenter et al.
UW-Madison Libraries provides free access to the online version of this textbook.
Works Cited
“Anesthesia (Guideline).” Anesthesia (Guideline)|Vertebrate Animal Research, https://animal.research.uiowa.edu/iacuc-guidelines-anesthesia.
Danneman PJ and Fish RE. Anesthesia and Analgesia in Laboratory Animals (Second Edition). Academic Press, 2008.
Flecknell PA. Laboratory Animal Anaesthesia Ed. 4. Academic Press, 2015.
Lamont L et al. Veterinary Anesthesia and Analgesia: The Sixth Edition of Lumb and Jones. Wiley Blackwell, 2024.
Paterson, E.A., O’Malley, C.I., Moody, C. et al. Development and validation of a cynomolgus macaque grimace scale for acute pain assessment. Sci Rep 13, 3209 (2023). https://doi.org/10.1038/s41598-023-30380-x
“Supplemental Anesthesia Training Resource.” Received by Rebecca Johnson, Supplemental Anesthesia Training Resource, 7 May 2020.
RARC’s “Overview of Anesthetic and Analgesic Procedures” must be read prior to accessing the below information.
Contact your RARC veterinarian prior to choosing an anesthetic, including agents that are NOT listed below.
Species-Specific Considerations
Pre-Anesthetic Considerations
- An acclimation period of at least 3 days is required prior to any procedure.
- Body weights must be obtained for appropriate drug dosing.
- Rabbits may become stressed by improper handling and use of volatile anesthetics during the induction phase of anesthesia, leading to respiratory and cardiac arrest.
- Some colonies have a prevalence of Pasturella multocida infection, contributing to respiratory issues during anesthesia. The occurrence of these problems may be limited by obtaining rabbits from disease-free sources, proper selection of anesthetics, and proper acclimation to handling prior to surgical procedures.
- Fasting is not required due to rabbits' inability to vomit. However, if endotracheal intubation occurs, the mouth should be cleaned out prior to intubation attempts with cotton tipped swabs.
- Rabbits are prone to ileus, so proper care should be demonstrated when handling them pre- and post-anesthesia, and feeding should occur as soon as possible after the procedure. Some pain medications can also play a role in ileus; address this with your veterinarian.
- Monitors, such as pulse oximetry and/or a doppler audible pulse monitor (see below), should be placed prior to induction (following pre-medication) to assess any cardiac arrhythmias during this time.
- Rabbits are sensitive to overheating. Therefore, regularly monitor temperature of the room that the rabbits are being housed in. The normal requirement for rabbit rooms is 64°F - 72°F.
- Subcutaneous (SC) injections are common. The preferred intramuscular (IM) injection site is within the lumbar epaxial musculature or the quadriceps muscle. The biceps femoris is not recommended due to the possibility of injecting near the sciatic nerve.
- An intravenous (IV) catheter should always be placed to administer additional anesthetic agents, emergency drugs, and/or fluids. The marginal/lateral ear vein is the preferred site; lidocaine-prilocaine (EMLA) cream can be placed 30-60 minutes prior to reduce pain with catheterization. Cephalic veins may also be used.
- Despite anesthetic chosen, sterile ophthalmic ointment should be applied to the eyes since blink reflexes are significantly reduced during anesthesia and sedation.
- It is very important to consult your RARC veterinarian when determining what anesthetic is best for your study.
- Neonatal rabbits require unique considerations; please consult your RARC veterinarian for specific recommendations.
Recommended Agents
Injectable Sedatives, Anesthetics, and Analgesics
Sedatives/Anesthetics
- Examples of sedative and anesthetic agents are included below, along with reversal agents.
- Detailed information concerning agents can be found at: Lamont et al, The Sixth Edition of Lumb & Jones.
- Premedication is common and is administered SC or IM prior to IV catheterization as discussed above.
- Premedication should be done in a familiar environment on a flat, sturdy surface with the rabbit well-restrained.
- Approximately 25-33% of rabbits have naturally occurring atropinases in their blood, which causes them to metabolize atropine (0.1-1 mg/kg SC, IM, IV) quickly. Repeated doses of atropine should be used or, alternatively, glycopyrrolate (0.01-0.1 mg/kg SC, IM, IV) may be administered ato increase heart rate if required.
- Supplemental oxygen should always be administered throughout sedation and/or anesthesia.
- Maropitant (1 mg/kg SC, IV) is frequently administered to aid with intestinal motility.
Sedatives Used in Rabbits
| Drug | Dosage/Route* | Duration | Comments |
| Acepromazine | 0.25-1 mg/kg SC, IM, IV | 45-60 minutes | Mild sedation only Vasodilation and hypotension |
| Midazolam | 0.25-2 mg/kg SC, IM, IV | 30-45 minutes | Mild to moderate sedation Reversed with flumazenil |
| Alpha-2 agonists: Dexmedetomidine OR Xylazine |
0.05-0.25 mg/kg SC, IM OR 1-5 mg/kg SC, IM |
30-45 minutes | Mild to profound sedation, based on dose Some analgesia Reversed with atipamezole |
| Alfaxalone | 6 mg/kg SC, IM | 30-40 minutes | Moderate sedation Apnea common |
* Subcutaneous (SC), intramuscular (IM), intravenous (IV). These can be used with other agents (such as opioids) to produce analgesia, profound sedation, or general anesthesia as below.
Injectable Anesthetics Used in Rabbits
| Drug | Dosage/Route* | Anesthetic Duration | Comments |
| Dissociative Combinations | |||
| Ketamine + midazolam |
10-40 mg/kg ketamine + 0.25-4 mg/kg midazolam SC, IM |
Up to 30 minutes | Light anesthetic plane Minimal analgesia Useful for non-invasive procedures Midazolam reversed with flumazenil |
| Ketamine + dexmedetomidine OR Ketamine + xylazine (less recommended) |
5-35 mg/kg ketamine + 0.02-0.20 mg/kg dexmedetomidine SC, IM OR 10-50 mg/kg ketamine + 3-5 mg/kg xylazine SC, IM |
Up to 90 minutes | RE-dose with 5-10 mg/kg ketamine only or use inhalant for additional anesthesia Cardiorespiratory depression; oxygen supplementation is recommended Dexmedetomidine/xylazine is reversed with atipamezole |
| Others | |||
| Alfaxalone + midazolam |
6 mg/kg alfaxalone + 1 mg/kg midazolam SC, IM |
Up to 60 minutes | Light anesthetic plane No analgesia Useful for non-invasive procedures Midazolam reversed with flumazenil Apnea common; oxygen supplementation is recommended |
| Alfaxalone + + dexmedetomidine |
6 mg/kg alfaxalone + 0.2 mg/kg dexmedetomidine SC, IM |
Up to 120 minutes | Apnea and cardiorespiratory depression; oxygen supplementation is recommended Dexmedetomidine is reversed with atipamezole |
| Propofol | 3-10 mg/kg IV slow bolus 0.1-0.8 mg/kg/min IV constant rate infusion (CRI) |
5-7 minutes | Titrate to effect for anesthetic induction or used as a CRI as part of partial intravenous anesthesia (PIVA) Apnea and cardiorespiratory depression; oxygen supplementation is recommended |
*Subcutaneous (SC), intramuscular (IM), intravenous (IV)
Reversal Agents Used in Rabbits
| Drug | Dosage/Route* | Reversal Category | Reversal For: |
| Atipamezole | 0.5-1 mg/kg SC, IM | Alpha-2 adrenergic receptors | Dexmedetomidine Xylazine |
| Flumazenil | 0.05-0.1 mg/kg SC, IP, IV | Benzodiazepines | Midazolam |
| Naloxone | 0.1-1 mg/kg SC, IP, IV | Opioids | Buprenorphine, morphine, etc. |
*Subcutaneous (SC), intramuscular (IM), intraperitoneal (IP), intravenous (IV)
Analgesics
- Examples of common analgesic agents are listed below. Others are available; please contact RARC veterinarians to discuss alternatives such as constant rate infusion including opioids (fentanyl: 2-20 mcg/kg/hr) and lidocaine (50-100 mcg/kg/min) IV.
- Unrelieved pain has profound physiologic consequences, which may alter research results.
- Pain assessment in rabbits consists of evaluating behavioral and physiologic parameters and use of validated pain scoring systems such as the Rabbit Pain Behavior Scale (RPBS; Pinho et al 2022).
- Pain scales include facial signs of pain and behavioral signs of pain including anxiety, apprehension, aggression, inactivity, reluctance to move, decreased appetite, tooth grinding, salivation, hunched posture, social isolation, decreased grooming, etc. The pain score and other signs should be documented in the record along with any analgesic intervention taken.
- The IACUC requires the use of preemptive analgesia (analgesics given prior to the first skin incision) for all survival surgical procedures unless scientifically justified.
- Preemptive analgesics may decrease the amount of required anesthetic drugs; doses of both injectable and inhalant agents should be adjusted accordingly.
Analgesic Agents Used in Rabbits
| Drug | Dosage/Route* | Frequency | Comments |
| Opioids | |||
| Buprenorphine (300 mcg/mL) | 0.05 mg/kg SC, IM, IV | 6-8 hours | Partial mu-opioid agonist Moderate analgesia |
| Extended-release buprenorphine (Ethiqa-XR®) | 0.15 mg/kg SC | Up to 72 hours | FDA indexed Partial mu-opioid agonist Onset = 30-60 minutes |
| Compounded extended-release buprenorphine (Buprenorphine Base-ER Lab) | 0.1 mg/kg SC | Up to 48 hours | Compounded Associated with skin nodules Partial mu-opioid agonist Less recommended |
| Hydromorphone | 0.05-0.2 mg/kg SC, IM, IV | 4-8 hours | Full mu-opioid agonist Profound analgesia |
| NSAIDs | |||
| Carprofen | 2-4 mg/kg SC, IM, PO | 24 hours | COX-2 selective |
| Meloxicam | 1 mg/kg SC, PO | 24 hours | COX-2 selective |
| Local Anesthetics** Doses must be calculated carefully due to toxicity potential with overdose (cardiac, CNS, etc.) |
|||
| Liposomal encapsulated bupivacaine (Nocita®)** | Up to 0.4 mL/kg intra-incisional infiltration | Up to 3 days | Must cover entire incision due to limited diffusion Favorable safety profile |
| Lidocaine** | Up to 6 mg/kg SC | 1-2 hours | Dilution of parent compound is frequently required Usually not recommended due to short duration of action |
| Bupivacaine** | Up to 2 mg/kg SC | 6-8 hours | Dilution of parent compound may be required Peak onset ~ 10 minutes |
*Subcutaneous (SC), intramuscular (IM), intraperitoneal (IP), intravenous (IV), oral (PO)
**Note: lidocaine and bupivacaine/Nocita® should not be mixed together.
Inhalant Anesthetics
Inhalant anesthetic delivery methods:
- Sedation must occur prior to mask induction with inhalant agents; these procedures are not recommended due to personnel exposure, slow induction times, and inability to control the airway.
- Agent-specific vaporizers are required, and excess gas must be scavenged.
- Inhalant should be turned off before the mask is removed or the entire procedure performed in a ducted fume hood or biosafety cabinet.
- Anesthetic maintenance using a nosecone or mask can be used.
- Endotracheal intubation is also used during anesthetic maintenace; RARC personnel should be contacted for more information concerning endotracheal intubation in rabbits.
- A carrier gas is required; usually it is 100% oxygen.
- Common settings:
- Induction: ~1-2 L/min; maintenance: ~0.5-1 L/min, depending on flow requirements of the system
- Common settings:
- Isoflurane and sevoflurane can both be used in rabbits. However, they require separate vaporizers due to different vapor pressures and the MAC values (see Overview of Anesthetic and Analgesia Procedures tab).
- Common vaporizer settings:
- Isoflurane - induction: ~2-4%, maintenance: 1-2%
- Sevoflurane - induction: ~4-6%, maintenance: ~3-4%
- Common vaporizer settings:
- Inhalants are profound cardiovascular and respiratory depressants; premedication is required to smooth induction/recovery and reduce the amount of inhalant required.
- Inhalants require proper delivery equipment and a scavenging system; RARC personnel can assist in choosing appropriate systems for your laboratory.
Endotracheal Intubation
- See Overview of Anesthetic and Analgesia Procedures tab for additional information.
- Rabbit endotracheal tubes are usually 2.0-3.5 mm and are often uncuffed.
- Rabbit intubation can be challenging. Intubation can be achieved by using a "blind" technique and capnometer, a modified otoscope, or a small endoscope.
- To perform the "blind" intubation, the rabbit is placed in sternal recumbency, and the handler extends and lifts the rabbit's head away from the table. Lidocaine is placed on the arytenoids. The endotracheal tube is inserted over the rabbit's tongue towards the larynx. The handler may listen for breathing sounds at the end of the tube. As the tube gets closer to the larynx, the breathing sounds will become louder. Condensation should start appearing at the end of the tube. An end-tidal CO2 monitor can be placed at the end of the endotracheal tube to guide placement. The tube can be passed through the larynx when the rabbit inhales. Correct placement of the tube is confirmed by continued presence of end-tidal CO2 and condensation in the tube.
- Please contact RARC veterinary staff if you have questions concerning this procedure.
Anesthetic Monitoring
Anesthetic monitoring techniques:
- Although constant vigilance is required during anesthesia (rabbits should never be unattended), recording vital signs every 5-10 minutes is recommended to immediately see values and to assess changes over time. RARC can provide you with an anesthetic record template if needed.
- Vital signs
- Cardiovascular system vital signs:
- Heart rate/pulse rate
- Can be taken using a pulse oximeter (see below), palpation of the heart, or stethoscope.
- Normal ~150-300 beats/minute, depending on anesthetic agents used.
- Arterial blood pressures
- Measured using oscillometric devices or by placement of direct lines into an artery.
- Heart rate/pulse rate
- Respiratory system vital signs:
- Respiratory rate
- Can be measured by visualizing the movement of the animal’s chest wall as it rises and falls or via the end-tidal carbon dioxide monitor (capnometer).
- Normal respiratory rates are species-dependent but typically are ~30-60 breaths/min in rabbits.
- Capnography
- Veterinary-specific monitors are available to measure end-tidal CO2
- Pulse oximetry
- Veterinary-specific monitors are available to measure the amount of oxygen saturation of hemoglobin in blood (normal = >95%) and pulse rate.
- Readings could be impaired by reduced pulsatile strength (hypotension, hypothermia, vasoconstriction, etc.).
- Respiratory rate
- Anesthetic depth
- Palpebral reflexes and limb withdrawal reflexes are performed to determine appropriate anesthetic depth prior to a procedure.
- Body temperature
- Rabbits can quickly become hypothermic or hyperthermic during anesthesia.
- Rectal thermometers specific to rabbits or implanted telemeters should be used.
- Temperature should not decrease below ~ 98°F throughout the procedure; circulating warm water blankets, forced warm air circulators, and warmed anesthetic circuits should be used.
- Excessive surgical clipping/scrubbing and drenching of rabbits with prepping agents should be minimized.
- Electrical blankets, rice bags, water bottles, and gloves are NOT allowed because they frequently produce burns.
- Pain scoring
- Validated pain scoring systems such as the Rabbit Pain Behavior Scale (RPBS) should be used and documented in the record.
- Analgesics must be administered as deemed necessary by intervention levels and per protocol or in discussion with the veterinary staff.
- Cardiovascular system vital signs:
Fluid Therapy
- Intra-operative anesthetic fluid rate should be ~10 mL/kg/hr IV or SC using balanced crystalloid solutions (Plasmalyte, Normosol, Lactated Ringer’s, etc.). Fluid rates and types should be adjusted based on individual animal needs.
- Voluntary fluid intake should be documented after the procedure into the recovery period.
- Once fully recovered, supplemental fluid can be given SC. Supplemental fluids should consist of a balanced crystalloid (Plasmalyte, Normosol, Lactated Ringer’s, etc.) and may be supplemented with other substances such as glucose.
Recovery
Factors that contribute to a successful recovery:
- Environment
- Recovery observations should be documented every 5-10 minutes until fully ambulating; a template can be provided by RARC if required.
- Recovery areas should be warm and quiet with dim yet sufficient lighting to appropriately observe the rabbit.
- Rabbits are sensitive to overheating. Therefore, regularly monitor the temperature of the rabbits' housing room. The normal requirement for rabbit rooms is 64°F - 72°F.
- For rabbits who are hypothermic, recover them in a warmer environment (80°F - 86°F) while using active thermal support. If the rabbit is extremely hypothermic, consider using an incubator until the rabbit returns to normal body temperature (101°F - 103°F). If an incubator is used, it is imperative to closely monitor the rabbit to avoid hyperthermia.
- Proper hydration and GI function
- Reduced fecal production can be due to pain, lack of fecal material in the GI tract, or ileus. If ileus is suspected, RARC veterinary personnel should be contacted.
- Supplemental fluid support (as described above), nutritional support (moist food, enriched gels, etc.) and/or active feeding techniques may be necessary and beneficial.
- Like fluid intake, food intake should be documented and monitored regularly.
Rabbit Validated Pain Scoring System
Pain Scale: Rabbit Pain Behavior Scale
|
ITEM |
Description |
Score |
|
Posture (check which of the following apply): |
||
|
1 |
A) Moves around normally and/or jumps |
|
|
B) Presents bipedal or quadrupedal position |
||
|
C) Walks at a very slow pace |
||
|
D) Lying down most of the time |
||
|
E) Does not move most of the time |
||
|
SCORING: Presence of the behavior(s) described in A and/or B only |
0 |
|
|
Presence of one of the behaviors described in C, D, or E |
1 |
|
|
Presence of two or more of the behaviors described in C, D, or E |
2 |
|
|
Activity: |
||
|
2 |
The rabbit moves normally and/or, when stationary, performs normal activity |
0 |
|
The rabbit moves little and does not perform normal activity |
1 |
|
|
The rabbit is immobile and does not perform normal activity |
2 |
|
|
Interaction and Appetite (check which of the following apply): |
||
|
3 |
A) Interacts with environmental enrichment objects |
|
|
B) Eats |
||
|
C) Sniffs the environment |
||
|
D) Presents self-cleaning behavior (grooming), except at the affected area |
||
|
SCORING: Presence of more than one of the behaviors described in A, B, C, or D |
0 |
|
|
Presence of one of the behaviors described in A, B, C, or D |
1 |
|
|
Absence of any of the behaviors described in A, B, C, or D |
2 |
|
|
Facial Expression (check which of the following apply): |
||
|
4 |
A) Keeps eyes wide open and ears erect all the time |
|
|
B) Keeps eyes semi-closed or closed at any moment |
||
|
C) Presents drooping ears at any moment |
||
|
SCORING: Presence of the expression described in A |
0 |
|
|
Presence of one of the expressions described in B or C |
1 |
|
|
Presence of the expressions described in B and C |
2 |
|
|
Attention to the affected area (check which of the following apply): |
||
|
5 |
A) Licks the affected area |
|
|
B) Presses the abdomen against the floor |
|
|
|
C) Keeps one limb suspended |
|
|
|
SCORING: The rabbit does not display any of the behaviors described in A, B, or C |
0 |
|
|
Presence of one of the behaviors described in A, B, or C |
1 |
|
|
Presence of more than one of the behaviors described in A, B, or C |
2 |
|
|
Miscellaneous Behaviors (check which of the following apply): |
||
|
5 |
A) Attempts to stand up, but remains lying down |
|
|
B) Rapid dorsal movement of the body (flinches) |
||
|
C) Retracts and closes the eyes (winces) |
||
|
D) Tremors |
||
|
SCORING: The rabbit does not present any of the behaviors described in A, B, C, or D |
0 |
|
|
Presence of one of the behaviors described in A, B, C, or D |
1 |
|
|
Presence of more than one of the behaviors described in A, B, C, or D |
2 |
|
Scale and Decision for Analgesic:
- Score total can range from 0 to 12
- Analgesic intervention given at a score ≥ 3
Additional Resources
Consult the RARC Veterinary Staff (vet@rarc.wisc.edu) if you have specific questions about the anesthetic procedures included in your IACUC protocol.
For assistance with anesthesia equipment set-up, please contact the RARC Trainers (trainer@rarc.wisc.edu).
Form templates to record anesthesia monitoring are available on the RARC website.
RARC loans out select anesthesia equipment to the UW-Madison research community for temporary use. Please visit the RARC website to find out what items are available to borrow.
Useful Text Resources:
Veterinary Anesthetic and Monitoring Equipment by Cooley and Johnson
UW-Madison Libraries provides free access to the online version of this textbook.
Veterinary Anesthesia and Analgesia: The Sixth Edition of Lumb and Jones by Lamont et al.
UW-Madison Libraries provides free access to the online version of this textbook.
Exotic Animal Formulary by Carpenter et al.
UW-Madison Libraries provides free access to the online version of this textbook.
Works Cited
“Anesthesia (Guideline).” Anesthesia (Guideline)|Vertebrate Animal Research, 10 May 2023. https://animal.research.uiowa.edu/iacuc-guidelines-anesthesia.
Danneman PJ and Fish RE. Anesthesia and Analgesia in Laboratory Animals (Second Edition). Academic Press, 2008.
Flecknell PA. Laboratory Animal Anaesthesia Ed. 4. Academic Press, 2015.
Lamont L et al. Veterinary Anesthesia and Analgesia: The Sixth Edition of Lumb and Jones. Wiley Blackwell, 2024.
“Supplemental Anesthesia Training Resource.” Received by Rebecca Johnson, Supplemental Anesthesia Training Resource, 7 May 2020.
RARC’s “Overview of Anesthetic and Analgesic Procedures” must be read prior to accessing the below information.
Contact your RARC veterinarian prior to choosing an anesthetic, including agents that are NOT listed below.
Species-Specific Considerations
Pre-Anesthetic Considerations
- An acclimation period of at least 3 days is required prior to any procedure.
- Body weights must be obtained for appropriate drug dosing.
- Fasting and water restriction is not routinely performed in rats due to their high metabolic rates and inability to vomit.
- Regardless of the anesthetic chosen, sterile ophthalmic ointment should be applied to the eyes since blink reflexes are significantly reduced during anesthesia and sedation.
- It is very important to consult your RARC veterinarian when determining what anesthetic is best for your study.
- Neonatal rats require unique considerations; please consult your RARC veterinarian for specific recommendations.
Recommended Agents
Injectable Sedatives, Anesthetics, and Analgesics
Sedatives/Anesthetics
- Examples of sedative and anesthetic agents are included below, along with reversal agents.
- Detailed information concerning agents can be found at: Lamont et al, The Sixth Edition of Lumb & Jones.
- For many researchers, ketamine combinations are the preferred choice of injectable anesthesia in rats.
- Classified as a dissociative anesthetic and an NMDA receptor antagonist; DEA schedule III-controlled substance.
- Ketamine alone produces muscle rigidity; therefore, it is commonly paired with other agents to produce a more comprehensive anesthetic experience for the animal.
- Popular ketamine combinations (+/- opioid):
- Ketamine/xylazine
- Ketamine/dexmedetomidine
- If an opioid is used as a premedication, lower doses of other agents are used.
- Injectable agents NOT recommended: alpha-chloralose, chloral hydrate, tribromoethanol.
Sedatives Used in Rats
| Drug | Dosage/Route* | Duration | Comments |
| Dissociative Combinations | |||
| Ketamine + midazolam |
75 mg/kg ketamine + 5 mg/kg midazolam IP |
20-30 minutes | Sedation only |
| Ketamine + acepromazine |
75 mg/kg ketamine + 2.5 mg/kg acepromazine IP |
20-30 minutes | Sedation only |
| Others | |||
| Alfaxalone | 20-30 mg/kg IP | 30-60 minutes | Sedation only No analgesia Apnea can be seen |
*Intraperitoneal (IP)
Injectable Anesthetics Used in Rats
| Drug | Dosage/Route* | Anesthetic Duration | Comments |
| Dissociative Combinations | |||
| Ketamine + xylazine |
40-80 mg/kg ketamine + 5-10 mg/kg xylazine IP |
20-30 minutes surgical anesthesia; up to 120 minutes of sedation |
Re-dose with 1/2 of ketamine dose only or use supplemental inhalant (~0.5-1%) for additional anesthesia Cardiorespiratory depression; oxygen supplementation is recommended Xylazine is reversed with atipamezole |
| Ketamine + dexmedetomidine |
75 mg/kg ketamine + 0.25-0.5 mg/kg dexmedetomidine IP |
20-30 minutes surgical anesthesia; up to 120 minutes of sedation |
Re-dose with 1/2 of ketamine dose only or use supplemental inhalant (~0.5-1%) for additional anesthesia Cardiorespiratory depression; oxygen supplementation is recommended Dexmedetomidine is reversed with atipamezole |
| Ketamine + acepromazine |
70-80 mg/kg ketamine + 2.5 mg/kg acepromazine IP |
20-30 minutes | Light sedation only Not reversable Potential for hypotension |
| Ketamine + xylazine + acepromazine |
31.25 mg/kg ketamine + 6.25 mg/kg xylazine + 1.25 mg/kg acepromazine IP |
20-40 minutes | Light to moderate sedation only Cardiorespiratory depression; oxygen supplementation is recommended Xylazine is reversed with atipamezole |
| Barbiturates | |||
| Pentobarbital | 40-50 mg/kg IP | 80-95 minutes | Cardiorespiratory depression and poor analgesia Narrow safety margin Only appropriate prior to terminal perfusions/euthanasia Not recommended for survival surgery |
| Others | |||
| Alfaxalone | 30 mg/kg IP | 30-60 minutes | Requires dexmedetomidine or other agent for surgical anesthesia Cardiorespiratory depression; oxygen supplementation is recommended Apnea is common |
| Propofol | 10 mg/kg IV | 5-7 minutes | Titrate to effect Cardiorespiratory depression; oxygen supplementation is recommended Apnea is common |
| Urethane | 1.5-1.8 g/kg IP, IV | 360-480 minutes | Minimal cardiorespiratory depression Carcinogenic - requires proper precautions and SOP for handling Terminal procedures only unless scientific justification provided |
*Intraperitoneal (IP), intravenous (IV)
Reversal Agents Used in Rats
| Drug | Dosage/Route* | Reversal Category | Reversal For: |
| Atipamezole | 0.1-1 mg/kg SC, IP | Alpha-2 adrenergic receptors | Dexmedetomidine Xylazine |
| Flumazenil | 0.1-10 mg/kg SC, IP, IV | Benzodiazepines | Midazolam |
| Naloxone | 0.1-1 mg/kg SC, IP, IV | Opioids | Buprenorphine, morphine, etc. |
*Subcutaneous (SC), intraperitoneal (IP), intravenous (IV)
Analgesics
- Examples of common analgesic agents are listed below. Others are available; please contact RARC veterinarians to discuss alternatives.
- Unrelieved pain has profound physiologic consequences, which may alter research results.
- Pain assessment in rats consists of evaluating behavioral and physiologic parameters and use of the Grimace Scale (see Analgesia Standard Treatment Guidelines for Laboratory Rats).
- In addition to facial signs of pain (Grimace Scale), behavioral signs of pain include reluctance to move, hunched posture, back arching, social isolation, decreased appetite, decreased grooming, decreased nest building, aggression, piloerection, self-aggression, and nasal/ocular porphyrin staining. Behavioral signs of pain should be documented in the record along with the Grimace Scale and any analgesic intervention taken.
- The IACUC requires the use of preemptive analgesia (analgesics given prior to the first skin incision) for all survival surgical procedures unless scientifically justified within an approved protocol.
- Preemptive analgesics may decrease the amount of required anesthetic drugs; doses of both injectable and inhalant agents should be adjusted accordingly.
Analgesic Agents Used in Rats
| Drug | Dosage/Route* | Frequency | Comments |
| Opioids | |||
| Buprenorphine (300 mcg/mL) | 0.01-0.05 mg/kg IP, SC | 6-8 hours | Partial mu-opioid agonist Pica is common |
| Extended-release buprenorphine (Ethiqa-XR®) | 0.65 mg/kg SC | Up to 72 hours | FDA indexed Partial mu-opioid agonist Pica is common |
| Compounded extended-release buprenorphine (Buprenorphine Base-ER Lab) | 1.2 mg/kg SC | Up to 48 hours | Compounded Associated with skin nodules Partial mu-opioid agonist Pica is common Less recommended |
| NSAIDs | |||
| Carprofen | 5 mg/kg SC | 24 hours | COX-2 selective |
| Meloxicam | 1-2 mg/kg SC, PO | 12-24 hours | COX-2 selective |
| Local Anesthetics** Doses must be calculated carefully due to toxicity potential with overdose (cardiac, CNS, etc.) |
|||
| Liposomal encapsulated bupivacaine (Nocita®)** | Up to 0.4 mL/kg intra-incisional infiltration | Up to 3 days | Must cover entire incision due to limited diffusion Favorable safety profile |
| Lidocaine** | Up to 6 mg/kg SC | 1-2 hours | Dilution of parent compound is frequently required Usually not recommended due to short duration of action |
| Bupivacaine** | Up to 2 mg/kg SC | 6-8 hours | Dilution of parent compound may be required Peak onset ~10 minutes |
*Subcutaneous (SC), intraperitoneal (IP), intravenous (IV), oral (PO)
**Note: lidocaine and bupivacaine/Nocita® should not be mixed together.
Inhalant Anesthetics
Inhalant anesthetic delivery methods:
- Induction chambers, along with nosecones and face masks, are commonly used in rats for induction and maintenance of general anesthesia.
- Endotracheal intubation is also used during anesthetic maintenance.
- A carrier gas is required; usually it is ~100% oxygen. Low flow systems are available.
- Common settings:
- Induction: ~0.5-1 L/min; maintenance: ~0.5-1 L/min for a standard, single non-rebreathing system. Different systems may have other carrier gas flow requirements - please check with the manufacturers or the RARC trainers.
- Common settings:
- Isoflurane and sevoflurane can both be used in rats. However, they require separate vaporizers due to different vapor pressures and the MAC values differ (see Overview of Anesthetic and Analgesia Procedures tab).
- Common vaporizer settings:
- Isoflurane - induction: ~2-4%, maintenance: ~1-2%
- Sevoflurane - induction: ~4-6%, maintenance: ~3-4%
- Common vaporizer settings:
- Inhalants are profound cardiovascular and respiratory depressants; premedication is required to smooth induction/recovery and to reduce the amount of inhalant required.
- Both isoflurane and sevoflurane require proper delivery equipment and a scavenging system; RARC personnel can assist in choosing appropriate systems for your laboratory.
- Inhalant anesthetics can be delivered either via an agent-specific vaporizer or drop box.
- Chamber induction (recommended)
- Controlled delivery of a specific inhalant concentration via a vaporizer; excess gas is scavenged.
- Inhalant should be turned off before chamber lid is opened to reduce personnel exposure, or entire procedure should be performed under a ducted fume hood or biosafety cabinet.
- Drop jar/Drop box method
- Not a recommended method due to lack of anesthetic concentration control and potential exposure of personnel to high inhalant concentrations.
- Used for brief and single uses only. Should not be used for any surgical or prolonged procedures.
- Gas must be scavenged. Procedure must be performed under a ducted fume hood or biosafety cabinet.
- Individuals working in accordance with wildlife protocols must ensure gas is being scavenged with charcoal filters and procedures must be performed in well-ventilated areas.
- Rats must not have contact with liquid anesthetic on a cotton ball, gauze, etc.
- The drop box must be cleaned after every animal to limit contamination.
- Chamber induction (recommended)
“Anesthesia (Guidelines)/ Vertebrate Animal Research”- University of Iowa
| Isoflurane Concentration in Drop Box | 1L | 2L | 3L | 4L | 5L |
| 1% | 0.05 mL | 0.10 mL | 0.15 mL | 0.20 mL | 0.26 mL |
| 2% | 0.10 mL | 0.20 mL | 0.31 mL | 0.41 mL | 0.51 mL |
| 3% | 0.15 mL | 0.31 mL | 0.46 mL | 0.61 mL | 0.77 mL |
| 4% | 0.20 mL | 0.41 mL | 0.61 mL | 0.82 mL | 1.02 mL |
| 5% | 0.26 mL | 0.51 mL | 0.77 mL | 1.02 mL | 1.28 mL |
Endotracheal Intubation
- See Overview of Anesthetic and Analgesia Procedures tab for additional information.
- Rodent endotracheal tubes are usually custom-made out of intravenous (IV) catheters and select cannulas. Intubation can be achieved by placing the rodent in dorsal recumbency or by using commercially available, specialized-sloped restraining devices.
- Please contact RARC veterinary staff if you have questions concerning this procedure.
Anesthetic Monitoring
Anesthetic monitoring techniques:
- Although constant vigilance is required during anesthesia (rats should never be unattended), recording vital signs every 5-10 minutes is recommended to immediately see values and to assess changes over time. RARC can provide you with an anesthetic record template if needed.
- Vital signs
- For smaller laboratory animals (e.g., rodents), required vital sign monitoring may be adapted from the signs discussed below:
- Cardiovascular system vital signs:
- Heart rate/pulse rate
- Can be taken using a pulse oximeter (see below), palpation of the heart, or stethoscope.
- Normal pulse is ~250-500 beats/minute, depending on anesthetic agents used.
- Arterial blood pressures
- Measured using rodent-specific oscillometric devices or by placement of direct lines into an artery.
- Heart rate/pulse rate
- Respiratory system vital signs:
- Respiratory rate
- Can be measured by visualizing the movement of the animal’s chest wall as it rises and falls or via the end-tidal carbon dioxide monitor (capnometer).
- Normal respiratory rates are species-dependent but typically are ~70-100 breaths/min in rats.
- Capnography
- Rodent-specific monitors are available to measure end-tidal CO2
- Pulse oximetry
- Rodent-specific monitors are available to measure the amount of oxygen saturation of hemoglobin in blood (normal = >95%) and pulse rate.
- Readings could be impaired by reduced pulsatile strength (hypotension, hypothermia, vasoconstriction, etc.).
- Respiratory rate
- Anesthetic depth
- Palpebral reflexes and limb withdrawal reflexes are performed to determine appropriate anesthetic depth prior to a procedure.
- Body temperature
- Rats can quickly become hypothermic during anesthesia but may become hyperthermic as well.
- Rectal thermometers specific to rats or implanted telemeters should be used.
- Temperature should not decrease below ~ 98°F throughout the procedure; circulating warm water blankets, forced warm air circulators, and warmed anesthetic circuits should be used.
- Excess surgical clipping/scrubbing and excessive drenching of rats with prepping agents should be minimized.
- Electrical blankets, rice bags, water bottles, and gloves are NOT allowed because they frequently produce burns.
- Pain scoring
- Validated pain scoring systems such as the Grimace Scale should be used and documented per guidelines on the Analgesia Standard Treatment Guidelines for Laboratory Rats tab.
- Analgesics must be administered as deemed necessary by intervention levels and per protocol or in discussion with the veterinary staff.
Fluid Therapy
- Intra-operative anesthetic fluid rate should be ~10 mL/kg/hr IV, SC, or IP using balanced crystalloid solutions (Plasmalyte, Normosol, Lactated Ringer’s, etc.). Fluid rates and types should be adjusted based on individual animal needs.
- Voluntary fluid intake should be documented after the procedure into the recovery period.
- Once fully recovered, supplemental fluids consisting of a balanced crystalloid (Plasmalyte, Normosol, Lactated Ringer’s, etc.) can be given SC or IP. Oral glucose and electrolyte-containing gels may also be used.
Recovery
Factors that contribute to a successful recovery:
- Environment
- Recovery observations should be documented every 5-10 minutes until fully ambulating; a template can be provided by RARC if required.
- Rats must not be moved to their normal housing cage until fully recovered. Premature transport to their home cage can result in the rodent inhaling bedding (such as corn cob), leading to airway obstruction or pneumonia. Instead, they should be transferred to a cage that has no bedding or a thin paper layer. Heat should be applied as needed (see above) underneath half of the recovery cage. This gives the animal the option and ability to self-regulate their temperature as they regain consciousness.
- Proper hydration and GI function
- Reduced fecal production can be due to pain, lack of fecal material in the GI tract, or ileus. If ileus is suspected, RARC veterinary personnel should be contacted.
- Supplemental fluid support (as described above), nutritional support (moist food, enriched gels, etc.), and/or feeding using oral gavage may be necessary and beneficial.
- Like fluid intake, food intake should be documented and monitored regularly.
- If buprenorphine is administered, pica can be observed; digestible bedding material may be required to prevent GI obstruction.
Rat Validated Pain Scoring System
Pain Scale: Rat Grimace Scale
|
ITEM |
Description |
Score |
|
Orbital Tightening: - Narrowing of the orbital area, a tightly closed eyelid, or an eye squeeze - An eye closure that reduces the eye opening by more than half should be coded as a “2” |
||
|
1 |
|
0 |
|
|
1 |
|
|
|
2 |
|
|
Nose/Cheek Flattening: - “No pain” = o Clear whisker bulge present at bridge of nose o Whisker pads are rounded and slightly puffed out o Clear crease between the pads and the cheeks - “In pain” = o Bridge of the nose flattens and elongates, causing whisker pads to flatten o Crease between the pads and the cheek is no longer present o In frontal headshots, the nose may appear narrower and longer |
||
|
2 |
|
0 |
|
|
1 |
|
|
|
2 |
|
|
Ear Changes (position, orientation, shape): - “No pain” = o Ears are roughly perpendicular to the head, facing forward, and angled slightly backward o Ears have a rounded shape - “In pain” = o Ears tend to fold or curl inwards and are angled forward o Curling of the ears tends to result in “pointed” shape of the ears - “Pronounced pain” = o Ears are angled outward and held close to 45° away from both the perpendicular axis and the nose o The space between the ears may appear wider relative to baseline |
||
|
3 |
|
0 |
|
|
1 |
|
|
|
2 |
|
|
Whisker Changes: - “No pain” = o Whiskers are relaxed and drooping slightly - “In pain” = o The whisker pad is contracted, causing the whiskers to bunch and be directed outwards, away from the face o Whiskers appear to be “standing on end” o Whiskers are closer together and are less distinct |
||
|
4 |
|
0 |
|
|
1 |
|
|
|
2 |
|
Scale and Decision for Analgesic:
- Score total can range from 0 to 8
- Calculate the mean of your results (total score/4)
- Analgesic intervention given at a score > 0.67
Sedative, Anesthetic and Analgesic Dilutions for Small Rats
Store drugs in a cool, dry place shielded from light. Diluted drugs must be discarded 30 days after dilution.
Acepromazine (10 mg/mL): Dilute to 1 mg/mL
Draw 1 mL acepromazine (10 mg/mL) into a sterile syringe and dispense into a sterile multi-dose vial. Add 9 mL of sterile diluent (usually 0.9% NaCl). Label vial with “acepromazine 1 mg/mL”, initials of person who prepared it, the preparation date, and the expiration date.
Bupivacaine (5 mg/mL): Dilute to 2.5 mg/mL
Draw 1 mL bupivacaine (5 mg/mL) into a sterile syringe and dispense into a sterile multi-dose vial. Add 1 mL of sterile diluent (usually 0.9% NaCl). Label vial with “bupivacaine 2.5 mg/mL”, initials of person who prepared it, the preparation date, and the expiration date.
Buprenorphine HCl (0.3 mg/mL): Dilute to 0.03 mg/mL
Draw 0.1 mL buprenorphine (0.3 mg/mL) into a sterile syringe and dispense into a sterile multi-dose vial. Add 0.9 mL of sterile diluent (usually 0.9% NaCl). Label vial with “buprenorphine 0.03 mg/mL”, initials of person who prepared it, the preparation date, and the expiration date.
Carprofen (50 mg/mL): Dilute to 2.5 mg/mL
Draw 0.2 mL carprofen (50 mg/mL) into a sterile syringe and dispense into a sterile multi-dose vial. Add 3.8 mL of sterile diluent (usually 0.9% NaCl). Label vial with “carprofen 2.5 mg/mL”, initials of person who prepared it, the preparation date, and the expiration date.
Dexmedetomidine (0.5 mg/mL): Dilute to 0.25 mg/mL
Draw 1 mL of dexmedetomidine (0.5 mg/mL) into a sterile syringe and dispense into a sterile multi-dose vial. Add 1 mL of sterile diluent (usually 0.9% NaCl). Label vial with “dexmedetomidine 0.25 mg/mL”, initials of person who prepared it, the preparation date, and the expiration date.
Alternatively, purchase the more dilute, commercial preparation of dexmedetomidine (0.1 mg/mL).
Ketamine (100 mg/mL): Dilute to 50 mg/mL
Draw 1 mL of ketamine (100 mg/mL) into a sterile syringe and dispense into a sterile multi-dose vial. Add 1 mL of sterile diluent (usually 0.9% NaCl). Label vial with “ketamine 50 mg/mL”, initials of person who prepared it, the preparation date, and the expiration date.
Midazolam (5 mg/mL): Dilute to 1 mg/mL
Draw 1 mL midazolam (5 mg/mL) into a sterile syringe and dispense into a sterile multi-dose vial. Add 4 mL of sterile diluent (usually 0.9% NaCl). Label vial with “midazolam 1 mg/mL”, initials of person who prepared it, the preparation date, and the expiration date.
Xylazine (20 mg/mL): Dilute to 2 mg/mL
Draw 1 mL xylazine (20 mg/mL) into a sterile syringe and dispense into a sterile multi-dose vial. Add 9 mL of sterile diluent (usually 0.9% NaCl). Label vial with “xylazine 2 mg/mL”, initials of person who prepared it, the preparation date, and the expiration date.
Additional Resources
Consult the RARC Veterinary Staff (vet@rarc.wisc.edu) if you have specific questions about the anesthetic procedures included in your IACUC protocol.
For assistance with anesthesia equipment set-up, please contact the RARC Trainers (trainer@rarc.wisc.edu).
Form templates to record anesthesia monitoring are available on the RARC website.
RARC loans out select anesthesia equipment to the UW-Madison research community for temporary use. Please visit the RARC website to find out what items are available to borrow.
Useful Text Resources:
Veterinary Anesthetic and Monitoring Equipment by Cooley and Johnson
UW-Madison Libraries provides free access to the online version of this textbook.
Veterinary Anesthesia and Analgesia: The Sixth Edition of Lumb and Jones by Lamont et al.
UW-Madison Libraries provides free access to the online version of this textbook.
Exotic Animal Formulary by Carpenter et al.
UW-Madison Libraries provides free access to the online version of this textbook.
Works Cited
“Anesthesia (Guideline).” Anesthesia (Guideline)|Vertebrate Animal Research, 10 May 2023. https://animal.research.uiowa.edu/iacuc-guidelines-anesthesia.
Danneman PJ and Fish RE. Anesthesia and Analgesia in Laboratory Animals (Second Edition). Academic Press, 2008.
Flecknell PA. Laboratory Animal Anaesthesia Ed. 4. Academic Press, 2015.
Lamont L et al. Veterinary Anesthesia and Analgesia: The Sixth Edition of Lumb and Jones. Wiley Blackwell, 2024.
“Supplemental Anesthesia Training Resource.” Received by Rebecca Johnson, Supplemental Anesthesia Training Resource, 7 May 2020.
RARC’s “Overview of Anesthetic and Analgesic Procedures” must be read prior to accessing the below information.
Contact your RARC veterinarian prior to choosing an anesthetic, including agents that are NOT listed below.
Species-Specific Considerations
Pre-Anesthetic Considerations
- An acclimation period of at least 3 days is required prior to any procedure.
- Body weights must be obtained for appropriate drug dosing.
- In ruminants, fasting has limited effect on the overall ruminal volume. However, small ruminants such as sheep and goats should be fasted (12-24 hrs) and adult cattle fasted up to 48 hrs to reduce bloat and particles in the regurgitative fluid; water should not be withheld. Although fasting cannot empty the rumen, it does help to reduce bloat during anesthesia (but does not eliminate it).
- Young ruminants (calves, lambs) are not usually fasted to reduce the risk of hypoglycemia.
- Pre-anesthetic drugs may be used to reduce the stress for the animal as they are moved from their pen or home environment to the procedure space and drugs can be administered via intramuscular (IM) injection or intravenous (IV) injection.
- IV access is relatively easily attained in the jugular vein for premedications or anesthetic induction agents. However, placement of an IV jugular catheter for drug administration is recommended. Other accessible veins include cephalic, auricular, saphenous, or coccygeal veins.
- Proper padding and positioning of ruminants during surgery is important to prevent neuromuscular damage. Ruminants should be placed on a flat surface with sufficient padding (≥ 1-2” thickness for calves, sheep, and goats; ≥ 4” thickness for adult cattle).
- Due to regurgitation, the oral cavity should be tipped downward to promote flow out of the mouth.
- General anesthesia can be achieved via IV injection in the jugular vein, tail/coccygeal vein, or anterior cephalic vein.
- Ruminants frequently hypoventilate under anesthesia due to drug-induced respiratory depression and the weight of the rumen pressing on the diaphragm. Mechanical ventilation is required for prolonged procedures with a peak inspiratory pressure ≤ 20 cm H2O.
- Despite the anesthetic chosen, sterile ophthalmic ointment should be applied to the eyes since blink reflexes are significantly reduced during anesthesia and sedation.
- It is very important to consult your RARC veterinarian when determining what anesthetic is best for your study.
- Neonatal ruminants require unique considerations; please consult your RARC veterinarian for specific recommendations.
Recommended Agents
Injectable Sedatives, Anesthetics, and Analgesics
Sedatives/Anesthetics
- Examples of sedative and anesthetic agents are included below, along with reversal agents.
- Detailed information concerning agents can be found at: Lamont et al, The Sixth Edition of Lumb & Jones.
- Premedication is common and is administered prior to IV anesthetic induction as discussed above.
- Premedication should be done in a familiar environment and animals left in a quiet, dimmed area to achieve maximal sedation prior to induction.
- Supplemental oxygen should always be administered throughout recumbent sedation and/or anesthesia.
- Procedures are frequently performed while the animal is standing under light to moderate sedation (using xylazine +/- butorphanol, for example) with a local anesthetic technique using lidocaine or bupivacaine (see below).
- Specific examples of standing protocols in adult cattle include:
- Xylazine (0.02 mg/kg) + butorphanol (0.02 mg/kg) IV for procedures such as liver or mammary biopsies.
- Ketamine “stuns” using IV protocols such as xylazine (up to 10 mg) + ketamine (up to 50 mg) + butorphanol (up to 5 mg) total per animal.
- Specifics for each drug are discussed below; veterinary personnel must be contacted before choosing a specific drug combination for your studies.
- Specific examples of standing protocols in adult cattle include:
Sedatives Used in Ruminants
| Drug | Dosage/Route* | Duration | Comments |
| Acepromazine | 0.005-0.03 mg/kg IM, IV | 45-60 minutes | Mild sedation only Vasodilation and hypotension Non-reversible |
| Midazolam | 0.1-1 mg/kg IM, IV | 15-30 minutes | Mild sedation to excitement Better effects in very young animals Frequently combined with other agents for seation (or anesthesia - see below) Reversed with flumazenil |
| Alpha-2 Agonists | |||
| Dexmedetomidine | Sheep/goats/calves: 0.005-0.01 mg/kg IV; 0.01-0.02 mg/kg IM Adult Cattle: 0.001-0.005 mg/kg IV; 0.005-0.015 mg/kg IM |
30-60 minutes | Mild to profound sedation, based on dose Mild analgesia Profound hypoxemia and potential pulmonary edema; respiratory monitoring is essential Cardiovascular depression Used with extreme caution in pregnant animals Reversed with atipamezole |
| Xylazine | Sheep/goats/calves: 0.05-0.1 mg/kg IV; 0.1-0.2 mg/kg IM Adult Cattle: 0.05-0.1 mg/kg IV; 0.1-0.3 mg/kg IM |
||
| Detomidine | Sheep/goats/calves: 0.003-0.02 mg/kg IV; 0.02-0.03 mg/kg IM Adult Cattle: 0.003-0.01 mg/kg IV; 0.01-0.02 mg/kg IM |
||
*Intramuscular (IM), intravenous (IV). These can be used with other agents (such as opioids and dissociatives) to produce analgesia and general anesthesia as below.
Injectable Anesthetics Used in Ruminants
| Drug | Dosage/Route* | Anesthetic Duration | Comments |
| Dissociative Combinations | |||
| Ketamine + midazolam |
3-5 mg/kg ketamine + 0.2-0.5 mg/kg midazolam IV |
Up to 30 minutes | Light anesthetic plane Minimal analgesia - frequently used with opioids Useful for non-invasive procedures Midazolam reversed with flumazenil |
| Ketamine + dexmedetomidine |
1-3 mg/kg ketamine + 0.005-0.01 mg/kg dexmedetomidine IM |
Up to 60 minutes | Light to moderate anesthetic plane Mild to moderate analgesia Profound hypoxemia and potential pulmonary edema; respiratory monitoring is essential Cardiovascular depression Used with extreme caution in pregnant animals IV administration is possible, but doses should be reduced and given "to effect" Dexmedetomidine/xylazine/detomidine reversed with atipamezole |
| Ketamine + xylazine |
1-3 mg/kg ketamine + 0.05-0.1 mg/kg xylazine IM |
||
| Ketamine + detomidine |
1-3 mg/kg ketamine + 0.005-0.01 mg/kg detomidine IM |
||
| Others | |||
| Total Intravenous Anesthesia (TIVA) "Double Drip": 5% Guaifenesin (1 L) + Ketamine (1000 mg/L) OR "Triple Drip": 5% Guaifenesin (1 L) + Ketamine (1000 mg/L) + Xylazine (5-10 mg/L) |
Delivered by "drip" or constant rate infusion (CRI) at 0.5-1 mL/kg/hr | Used only up to 60 minutes | Administered to effect during anesthetic maintenance Light to moderate anesthetic plane Hypoxemia common; oxygen supplementation is recommended Low therapeutic index (therapeutic dose:lethal dose = 1:3); do not overdose Addition of xylazine in "Triple Drip" is associated with cardiovascular depression and potential pulmonary edema |
| Propofol | 1-4 mg/kg IV slow bolus 0.2-0.8 mg/kg/min IV constant rate infusion (CRI) |
5-7 minutes | Titrate to effect for anesthetic induction or used as a CRI as part of partial intravenous anesthesia (PIVA) Cardiorespiratory depression; oxygen supplementation is recommended Apnea is common |
| Alfaxalone | 0.5-3 mg/kg IV | 5-7 minutes | Titrate to effect for anesthetic induction Cardiorespiratory depression although heart rate may be better maintained than with propofol; oxygen supplementation is recommended Apnea is common |
*Intramuscular (IM), intravenous (IV). These can be used with opioids to produce analgesia with profound sedation or general anesthesia as below.
Reversal Agents Used in Ruminants
| Drug | Dosage/Route* | Reversal Category | Reversal For: |
| Atipamezole | 0.1-0.2 mg/kg SC, IM | Alpha-2 adrenergic receptors | Dexmedetomidine Xylazine Detomidine |
| Flumazenil | 0.02-0.08 mg/kg SC, IM, IV | Benzodiazepines | Midazolam Zolazepam |
| Naloxone | 0.1-1 mg/kg SC, IM, IV | Opioids | Buprenorphine, morphine, etc. |
*Subcutaneous (SC), intramuscular (IM), intravenous (IV)
Analgesics
- Examples of common analgesic agents are listed below. Others are available; please contact RARC veterinarians to discuss alternatives such as gabapentin, acetaminophen, etc.
- Unrelieved pain has profound physiologic consequences, which may alter research results.
- Pain assessment in ruminants consists of evaluating behavioral and physiologic parameters and use of validated pain scoring systems such as the Unesp-Botucatu Sheep Composite Acute Pain Scale (UPAPS), the Unesp-Botucatu Goat Composite Acute Pain Scale (UGAPS) or the Unesp-Botucatu Unidimensional Cattle Composite Acute Pain Scale (UPAPS) (Silva et al 2020; Fonseca et al 2023; de Oliveira et al 2014).
- Pain scales include abnormal gait and posture, interactions with surroundings, reluctance to move, activity, social isolation, attention to the painful area, tail wagging, licking, kicking, recumbency, and appetite. The pain score and other signs should be documented in the record along with any analgesic intervention taken.
- The IACUC requires the use of preemptive analgesia (analgesics given prior to the first skin incision) for all survival surgical procedures unless scientifically justified.
- Pre-emptive analgesics may decrease the amount of required anesthetic drugs; doses of both injectable and inhalant agents should be adjusted accordingly.
Analgesic Agents Used in Ruminants
| Drug | Dosage/Route* | Frequency | Comments |
| Opioids/Sodium Channel Blockers/NMDA Antagonists | |||
| Buprenorphine (300 mcg/mL) or high concentration buprenorphine (Simbadol™; 1.8 mg/mL) | 0.006-0.01 mg/kg IM, IV | 6-8 hours | Partial mu-opioid agonist Moderate analgesia |
| Morphine | 0.5-1 mg/kg IM, slow IV | 4-8 hours | Full mu-opioid agonist Profound analgesia |
| Butorphanol | Sheep/goats/calves: 0.1-0.5 mg/kg IM, IV Adult Cattle: 0.02-0.05 mg/kg IM, IV |
1-2 hours | Mu-opioid antagonist; kappa-opioid agonist Mild, short analgesia |
| Fentanyl | 2-5 mcg/kg bolus IV 2-20 mcg/kg/hr IV OR 1-2 mcg/kg/hr TD |
CRI OR Patch |
Full-mu agonist Profound analgesia Patch takes up to 18 hr for full effect and can be variable; up to 72 hr duration |
| Lidocaine | 0.5-2 mg/kg bolus IV 50-75 mcg/kg/min IV |
CRI | Sodium channel blockade Anti-inflammatory, reduces inhalant required |
| Ketamine | 0.25-1 mg/kg bolus SC, IV 2-20 mcg/kg/min IV |
CRI | Excellent somatic analgesia Reduces inhalant required |
| NSAIDs | |||
| Carprofen | 4 mg/kg SC | 24 hours | COX-2 selective |
| Meloxicam | 1-2 mg/kg SC, PO | 24 hours | COX-2 selective |
| Phenylbutazone | 5 mg/kg PO | 12 hours | Non-selective Animals CANNOT enter the food chain |
| Flunixin Meglumine | 1.1-2.2 mg/kg IV | 12-24 hours | Non-selective |
| Local Anesthetics** Doses must be calculated carefully due to toxicity potential with overdose (cardiac, CNS, etc.) |
|||
| Liposomal encapsulated bupivacaine (Nocita®)** | Up to 0.4 mL/kg intra-incisional infiltration | Up to 3 days | Must cover entire incision due to limited diffusion Favorable safety profile |
| Lidocaine** | Up to 6 mg/kg SC | 1-2 hours | Short duration of action - best for minimally painful procedure |
| Bupivacaine** | Up to 2 mg/kg SC | 6-8 hours | Peak onset ~10 minutes |
*Subcutaneous (SC), intraperitoneal (IP), intravenous (IV), oral (PO), transdermal (TD)
**Lidocaine and bupivacaine/Nocita® should not be mixed together
Inhalant Anesthetics
Inhalant anesthetic delivery methods:
- General anesthesia should be induced using an injectable agent as described above.
- Mask induction is not recommended due to personnel exposure, slow induction times, danger to personnel when restraining large animals, and inability to control the airway. If deemed necessary in young ruminants, sedation should precede mask induction and the diaphragm within the mask must result in a tight seal around the nose/mouth.
- Agent-specific vaporizers are required, and excess gas must be scavenged.
- Inhalant should be turned off before mask is removed.
- Anesthetic maintenance using a mask can be performed but is not recommended due to personnel exposure, inability to protect the airway from regurgitation, and inability to control ventilation.
- Endotracheal intubation is recommended during anesthetic maintenance; RARC personnel should be contacted for more information concerning endotracheal intubation in ruminants.
- A carrier gas is required; usually it is ~100% oxygen.
- Common settings:
- Immediately following induction: ~2 L/min; maintenance: ~1 L/min.
- Common settings:
- Isoflurane and sevoflurane can both be used in ruminants. However, they require separate vaporizers due to different vapor pressures and the MAC values (see Overview of Anesthetic and Analgesia Procedures tab).
- Common vaporizer settings:
- Isoflurane: immediately following induction ~2-4%, maintenance ~1-2%
- Sevoflurane: immediately following induction ~4-6%, maintenance ~3-4%
- Common vaporizer settings:
- Inhalants are profound cardiovascular and respiratory depressants; premedication is required to smooth induction/recovery and reduce amount of inhalant required.
- Inhalants require proper circle/rebreathing and scavenging systems; RARC personnel can assist in choosing appropriate systems for your laboratory.
Endotracheal Intubation
- See Overview of Anesthetic and Analgesia Procedures tab for additional information. Please consult with RARC personnel if you are performing tracheal intubation in ruminants.
- Orotracheal intubation can be performed using a fiberoptic laryngoscope or a non-metal stylet or bougie (small ruminants), or blindly with manual palpation (larger ruminants).
- Ruminant endotracheal tubes depend on the age/size of the animal. Adult sheep, goat and calf tubes are usually 7.0-10.0 mm; whereas, adult cattle tubes can approach 26 mm in diameter. Neonatal sheep and goats require much smaller tubes and can be as small as 4.0 mm.
- Ruminants should be placed in sternal recumbency with the head/neck extended. The laryngoscope should be placed on the base of the tongue – not the epiglottis – to visualize the entrance to the trachea. In small ruminants, 2% lidocaine (~ 1 mL in an adult) is placed on the arytenoid cartilages. The endotracheal tube cuff should be lubricated with a sterile, water-soluble lubricant to form an appropriate seal. An introducer may be used; these are non-metal stylets or bougies with blunt ends that are inserted inside the trachea over which the endotracheal tube is passed. If an introducer is not used, the endotracheal tube is placed curved side down to pass into the trachea due to laryngeal/tracheal diverticula. In large ruminants, manual placement of the endotracheal tube with palpation of the epiglottis and rima glottidis should be performed.
- The endotracheal tube cuff should be inflated immediately to reduce aspiration of ruminal contents.
- If regurgitation is noted, the oral cavity should be tipped down to promote drainage.
- Please contact RARC if you have questions concerning this procedure.
Anesthetic Monitoring
Anesthetic monitoring techniques:
- Although constant vigilance is required during anesthesia (pigs should never be unattended), recording vital signs every 5-10 minutes is recommended to immediately see values and to assess changes over time. RARC can provide you with an anesthetic record template if needed.
- Vital signs
- Cardiovascular system vital signs:
- Heart rate/pulse rate
- Can be taken using a pulse oximeter (see below), palpation of the heart or stethoscope.
- Normal ~80-150 beats/minute (sheep/goats/calves); 70-100 (adult cattle), depending on anesthetic agents used.
- Arterial blood pressures
- Measured using oscillometric devices or by placement of direct lines into an artery.
- Mean arterial pressure should always be kept above ~70 mmHg to ensure organ perfusion; ruminants frequently have mean arterial pressures = 75-100 mmHg under general anesthesia, higher than many other species.
- Respiratory system vital signs:
- Respiratory rate
- This can be measured by visualizing the movement of the animal’s chest wall as it rises and falls or via the end-tidal carbon dioxide monitor (capnometer).
- Normal respiratory rates typically are ~20-40 breaths/min.
- Capnography
- Veterinary-specific monitors measure end-tidal CO2 levels and produce capnograph waves. These are useful in detecting abnormalities in ventilation and appropriate tracheal intubation.
- This is an essential monitor in any mechanically-ventilated patient.
- Pulse oximetry
- Veterinary-specific monitors are available to measure the amount of oxygen saturation of hemoglobin in blood (normal = >95%) and pulse rate.
- Readings could be impaired by reduced pulsatile strength (hypotension, hypothermia, vasoconstriction, etc.).
- Respiratory rate
- Anesthetic depth
- Jaw tone, palpebral reflexes and limb withdrawal reflexes are performed to determine appropriate anesthetic depth prior to a procedure.
- Body temperature
- Ruminants can become hypothermic or hyperthermic during anesthesia (normal = 101.5-103.5 °F).
- Rectal or esophageal thermometers or implanted telemeters should be used.
- Temperature should not decrease lower than ~ 98°F throughout the procedure; circulating warm water blankets, forced warm air circulators and warmed anesthetic circuits should be used.
- Surgical clipping and scrubbing or prepping agents should be minimized.
- Electrical blankets, rice bags, water bottles, and gloves are NOT allowed because they frequently produce burns.
- Pain scoring
- Validated pain scoring systems such as the Unesp-Botucatu Sheep Composite Acute Pain Scale (UPAPS), the Unesp-Botucatu Goat Composite Acute Pain Scale (UGAPS), or the Unesp-Botucatu Unidimensional Cattle Composite Acute Pain Scale (UPAPS) (Silva et al 2020; Fonseca et al 2023; de Oliveira et al 2014) should be used and documented in the record.
- Analgesics should be administered as deemed necessary by intervention levels and per protocol or in discussion with RARC veterinary personnel.
- Heart rate/pulse rate
- Cardiovascular system vital signs:
Fluid Therapy
- Intra-operative anesthetic fluid rate should be ~3-5 mL/kg/hr IV using balanced crystalloid solutions (Plasmalyte, Normosol, Lactated Ringer’s, etc.). Fluid rates and types should be adjusted based on individual animal needs.
- Voluntary fluid intake should be documented after the procedure into the recovery period.
- Once fully recovered, supplemental fluid can be given IV. Supplemental fluids should consist of a balanced crystalloid (Plasmalyte, Normosol, Lactated Ringer’s, etc.) and may be supplemented with other substances such as glucose.
Recovery
Factors that contribute to a successful recovery:
- Environment
- Recovery observations should be documented every 5-10 minutes until fully ambulating; a template can be provided by RARC if required.
- Recovery areas should be warm and quiet with dim, yet sufficient lighting to appropriately observe the animal.
- If adequate room temperatures cannot be achieved (³ ~ 60°F adults; ³ ~ 70°F neonates), the incorporation of supplemental heat sources should be used as above.
- A recovery pen with proper padding on the walls and floor (when they are in recumbency) is required. An impermeable surface or rubber mat will help as they regain footing and ambulation.
- Straw or hay bales can be used to keep animals in sternal recumbency; this position promotes eructation which can aid in bloat.
- Ruminants should be extubated at the return of the swallow reflex and when they can independently maintain sternal recumbency’ the endotracheal tube cuff should remain fully or partially inflated during extubation to remove any foreign material from the trachea.
- Proper hydration and GI function
- Supplemental fluid support (as described above) and nutritional support (moist food, enriched food, etc.) and/or active feeding techniques may be necessary and beneficial. Palatable food should be offered when ruminants are ambulating and have fully regained their swallow reflex to reduce GI stasis.
- Like fluid intake, food intake should be documented in the record and monitored regularly.
Ruminant Validated Pain Scoring Systems
Anesthetic Emergencies
Cattle
Pain scale: Unesp-Botucatu Cattle Pain Scale
|
ITEM |
Description |
Score |
|
Locomotion: |
||
|
1 |
Walking with no obviously abnormal gait |
0 |
|
Walking with restriction, may be with hunched back and/or short steps |
1 |
|
|
Reluctant to stand up, standing up with difficulty, or not walking |
2 |
|
|
Interactive Behavior: |
||
|
2 |
Active; attention to tactile and/or visual and/or audible environmental stimuli; can interact with and/or accompany the group |
0 |
|
Apathetic; may remain close to other animals, but interacts little when stimulated |
1 |
|
|
Apathetic; may be isolated or may not accompany the other animals; does not react to tactile, visual, and/or audible environmental stimuli |
2 |
|
|
Activity: |
||
|
3 |
Moves normally |
0 |
|
Restless, moves more than normal, or lies down and stands up with frequency |
1 |
|
|
Moves less frequently in the pasture or only when stimulated |
2 |
|
|
Appetite: |
||
|
4 |
Normal appetite and/or rumination |
0 |
|
Decreased appetite |
1 |
|
|
No appetite |
2 |
|
|
Check which of the following apply: |
||
|
5 |
Wagging the tail abruptly and repeatedly |
|
|
Licking the affected area |
||
|
Moves and arches the back when in standing posture |
||
|
Kicking/foot stamping |
||
|
Hindlimbs extended caudally when in standing posture |
||
|
Head below the line of the spinal column |
||
|
Lying down in ventral recumbency with full or partial extension of one or both hindlimbs |
||
|
Lying down with the head on or close to the ground |
||
|
Extending the neck and body forward when lying in ventral recumbency |
||
|
SCORING: All above behaviors in Item 5 are absent |
0 |
|
|
Presence of one of the above behaviors in Item 5 |
1 |
|
|
Presence of two or more of the above behaviors in Item 5 |
2 |
|
Scale and Decision for Analgesic:
- Score total can range from 0 to 10
- Analgesic intervention given at a score ≥ 4
Goats
Pain scale: Unesp-Botucatu Goat Pain Scale
|
ITEM |
Description |
Score |
|
Posture (check which of the following apply): |
||
|
1 |
A) Normal or jumps or it is in a bipedal position |
|
|
B) Not normal or does not jump or is not in a bipedal position |
||
|
C) Unstable |
||
|
D) Difficulty or attempting to lie down |
||
|
SCORING: Presence of the behavior described in A only |
0 |
|
|
Presence of one of the behaviors described in B, C, or D |
1 |
|
|
Presence of two of the behaviors described in B, C, or D |
2 |
|
|
Presence of the three behaviors described in B, C, and D |
3 |
|
|
Locomotion (check which of the following apply): |
||
|
2 |
A) Walks or runs |
|
|
B) Does not walk or run |
|
|
|
C) Lying down |
|
|
|
D) Motionless |
|
|
|
SCORING: Presence of the behavior described in A only |
0 |
|
|
Presence of one of the behaviors described in B, C, or D |
1 |
|
|
Presence of two of the behaviors described in B, C, or D |
2 |
|
|
Presence of the three behaviors described in B, C, and D |
3 |
|
|
Attitude: |
||
|
3 |
Vocalizes |
0 |
|
Does not vocalize |
1 |
|
|
Interaction: |
||
|
4 |
Interacts with another goat (by smelling) or with the environment |
0 |
|
Does not interact with another goat (by smelling) or with the environment |
1 |
|
|
Attention to the affected area (check which of the following apply): |
||
|
5 |
A) Looks at or licks |
|
|
B) Stamps pelvic limb |
||
|
SCORING: None of the above behaviors in Item 5 are present. |
0 |
|
|
Presence of one of the behaviors described in A or B |
1 |
|
|
Presence of both of the behaviors described in A and B |
2 |
|
Scale and Decision for Analgesic:
- Score total can range from 0 to 10
- Analgesic intervention given at a score ≥ 3
Sheep
Pain Scale: Unesp-Botucatu Sheep Pain Scale
|
ITEM |
Description |
Score |
|
Interaction: |
||
|
1 |
Active, attentive to the environment, interacts and/or follows other animals, or sleeps |
0 |
|
Apathetic; may remain close to other animals, but interacts little |
1 |
|
|
Very apathetic; isolated or not interacting with other animals, not interested in the environment |
2 |
|
|
Locomotion: |
||
|
2 |
Moves about freely, without altered locomotion, or sleeps. When stopped, the pelvic limbs are parallel to the thoracic limbs. |
0 |
|
Moves about with restriction and/or lameness. When stopped, the thoracic or pelvic limbs may be more open and further back than normal. |
1 |
|
|
Difficulty and/or reluctant to get up and/or not moving and/or walking abnormally and/or limping; may lean against a surface. |
2 |
|
|
Head position: |
||
|
3 |
Head above the withers or eating or sleeping |
0 |
|
Head at the height of the withers |
1 |
|
|
Head below the withers (except when eating) |
2 |
|
|
Posture (check which of the following apply): |
||
|
4 |
A) Arched back |
|
|
B) Extends the head and neck |
||
|
C) Lying down with head resting on the ground or close to the ground |
||
|
D) Moves the tail quickly and repeatedly (except when breastfeeding) and/or keeps the tail straight (except when defecating or urinating) |
||
|
SCORING: None of the above behaviors in Item 4 are present. |
0 |
|
|
Presence of one of the behaviors described in A, B, C, or D |
1 |
|
|
Presence of two or more of the behaviors described in A, B, C, or D |
2 |
|
|
Activity: |
||
|
5 |
Moves normally or sleeps |
0 |
|
Restless, moves more than normal, or lies down and gets up frequently |
1 |
|
|
Moves less frequently, or only when stimulated using a stick, or does not move |
2 |
|
|
Appetite: |
||
|
6 |
Normal appetite and/or rumination present |
0 |
|
Decreased appetite |
1 |
|
|
No appetite |
2 |
|
Scale and Decision for Analgesic:
- Score total can range from 0 to 12
- Analgesic intervention given at a score ≥ 4
Additional Resources
Consult the RARC Veterinary Staff (vet@rarc.wisc.edu) if you have specific questions about the anesthetic procedures included in your IACUC protocol.
For assistance with anesthesia equipment set-up, please contact the RARC Trainers (trainer@rarc.wisc.edu).
Form templates to record anesthesia monitoring are available on the RARC website.
RARC loans out select anesthesia equipment to the UW-Madison research community for temporary use. Please visit the RARC website to find out what items are available to borrow.
Useful Text Resources:
Veterinary Anesthetic and Monitoring Equipment by Cooley and Johnson
UW-Madison Libraries provides free access to the online version of this textbook.
Veterinary Anesthesia and Analgesia: The Sixth Edition of Lumb and Jones by Lamont et al.
UW-Madison Libraries provides free access to the online version of this textbook.
Exotic Animal Formulary by Carpenter et al.
UW-Madison Libraries provides free access to the online version of this textbook.
Works Cited
“Anesthesia (Guideline).” Anesthesia (Guideline)|Vertebrate Animal Research, 10 May 2023. https://animal.research.uiowa.edu/iacuc-guidelines-anesthesia.
Danneman PJ and Fish RE. Anesthesia and Analgesia in Laboratory Animals (Second Edition). Academic Press, 2008.
de Oliveira FA, Luna SP, do Amaral JB, Rodrigues KA, Sant'Anna AC, Daolio M, Brondani JT. 2014. Validation of the UNESP-Botucatu unidimensional composite pain scale for assessing postoperative pain in cattle. BMC Vet Res. 2014 Sep 6;10:200. doi: 10.1186/s12917-014-0200-0.
Flecknell PA. Laboratory Animal Anaesthesia Ed. 4. Academic Press, 2015.
Fonseca MW, Trindade PHE, Pinho RH, Justo AA, Tomacheuski RM, Silva NEOFD, Gonçalves HC, Luna SPL. 2023. Development and Validation of the Unesp-Botucatu Goat Acute Pain Scale. Animals (Basel). Jun 28;13(13):2136. doi: 10.3390/ani13132136.
Lamont L et al. Veterinary Anesthesia and Analgesia: The Sixth Edition of Lumb and Jones. Wiley Blackwell, 2024.
Silva NEOF, Trindade PHE, Oliveira AR, Taffarel MO, Moreira MAP, Denadai R, Rocha PB, Luna SPL. 2020. Validation of the Unesp-Botucatu composite scale to assess acute postoperative abdominal pain in sheep (USAPS). PLoS One. Oct 14;15(10):e0239622. doi: 10.1371/journal.pone.0239622. eCollection 2020.
“Supplemental Anesthesia Training Resource.” Received by Rebecca Johnson, Supplemental Anesthesia Training Resource, 7 May 2020.
RARC’s “Overview of Anesthetic and Analgesic Procedures” must be read prior to accessing the below information.
Contact your RARC veterinarian prior to choosing an anesthetic, including agents that are NOT listed below.
Species-Specific Considerations
Pre-Anesthetic Considerations
- An acclimation period of at least 3 days is required prior to any procedure. Pigs transferred between campus facilities do not require an acclimation period; although, it is recommended.
- Body weights must be obtained for appropriate drug dosing.
- Body size and conformation vary greatly between pigs. Use of restraint devices such as “pig boards” or slings may be recommended.
- Swine may become stressed and frequently vocalize when handled. Premedications are recommended to reduce stress.
- Acclimatation and environmental conditioning is essential to improve sedation, anesthetic procedures and outcomes. Animals accustomed to handling and personnel require reduced drug doses, achieve desired levels of sedation/anesthesia and are generally less stressed.
- Pig skin and subcutaneous adipose tissue are quite thick; appropriately sized needles are required for IM injections. For example, 18 gauge, 1 ½ inch needles for adults; 20 gauge, 1 ½ inch needles for young or thin pigs.
- Pigs have few superficial veins for IV access. Thus, many premedications are administered IM. The preferred site is in the cervical musculature caudal to the ear.
- IV catheterization or injection frequently occurs in the auricular (ear) veins although the cephalic and saphenous veins can also be used. For multiple IV injections, implantation of long-term jugular catheters or vascular access ports (VAPS) should be performed.
- Auricular IV catheters are usually 18-22 gauge, depending on ear size and can be taped in using a cotton roll on the inside of the ear, staple in using a “butterfly” tape or adhered to the skin using a sticky, non-porous dressing such as Tegaderm.
- Food should be withheld for ~ 12-18 hr to reduce regurgitation and/or vomiting. However, gastric emptying times vary significantly between pigs and an empty stomach is not guaranteed. Water should not be withheld.
- Maropitant (1 mg/kg) SC or slow IV may be administered to reduce vomiting.
- Swine frequently have vasospasm, cardiac arrhythmias and pulmonary damage; cardiopulmonary monitoring is required and peak inspired pressures are kept < 20 cm H2O if mechanically ventilated.
- Pigs are susceptible to Malignant Hyperthermia, a pharmacogenetic disease characterized by a hypermetabolic state in muscles induced by stress or pharmacologic agents such as inhalants. Initial signs include increased end-tidal CO2, lactate and potassium levels, and body temperature, with a decreased pH (acidosis). Genetically susceptible pigs should be treated with dantrolene. The onset is usually seen immediately after anesthetic induction.
- Pigs may also show signs of hyperthermia and increased lactate associated with the procedure. This distinct condition is usually seen during anesthetic recovery and is treated with cooling therapies such as cool water baths, IV fluid therapy, application of fans, and decreasing room temperature.
- Despite anesthetic chosen, sterile ophthalmic ointment should be applied to eyes since blink reflexes are significantly reduced during anesthesia and sedation.
- It is very important to consult your RARC veterinarian when determining what anesthetic is best for your study.
- Neonatal pigs require unique considerations; please consult your RARC veterinarian for specific recommendations.
Recommended Agents
Injectable Sedatives, Anesthetics and Analgesics
Sedatives/Anesthetics
- Examples of sedative and anesthetic agents are included below, along with reversal agents.
- Detailed information concerning agents can be found at: Lamont et al, The Sixth Edition of Lumb & Jones.
- Premedication is common and is administered IM prior to IV catheterization as discussed above.
- Premedication should be done in a familiar environment and pigs left in a quiet, dimmed area to achieve maximal sedation.
- Supplemental oxygen should always be administered throughout sedation and/or anesthesia.
Sedatives Used in Swine
| Drug | Dosage/Route* | Duration | Comments |
| Acepromazine | 0.01-0.05 mg/kg IM, IV | 45-60 minutes | Mild sedation only Vasodilation and hypotension |
| Midazolam | 0.1-1 mg/kg IM, IV 0.1-1 mg/kg/hr IV constant rate infusion (CRI) |
30-60 minutes | Mild to moderate sedation Reversed with flumazenil |
| Alpha-2 agonists: Dexmedetomidine OR Xylazine OR Detomidine |
0.01-0.04 mg/kg IM, IV OR 1-3 mg/kg IM, IV OR 0.05-0.1 mg/kg IM, IV |
30-60 minutes | Mild to profound sedation, based on dose Some analgesia Reversed with atipamezole |
* Intramuscular (IM), intravenous (IV). These can be used with other agents (such as opioids and dissociatives) to produce analgesia, profound sedation, or general anesthesia as below.
Injectable Anesthetics Used in Swine
| Drug | Dosage/Route* | Anesthetic Duration | Comments |
| Dissociative Combinations | |||
| Ketamine + midazolam |
10-30 mg/kg ketamine + 0.1-1 mg/kg midazolam IM |
Up to 30 minutes | Light anesthetic plane Minimal analgesia - frequently used in combination with an opioid Useful for non-invasive procedures Midazolam reversed with flumazenil |
| Ketamine + dexmedetomidine OR Ketamine + xylazine OR Ketamine + detomidine |
10-30 mg/kg ketamine + 0.01-0.02 mg/kg dexmedetomidine IM OR 10-30 mg/kg ketamine + 1-2 mg/kg xylazine IM OR 10-30 mg/kg ketamine + 0.05 mg/kg detomidine IM |
Up to 60 minutes | Light to moderate anesthetic plane Cardiorespiratory depression; oxygen supplementation is recommended Dexmedetomidine/xylazine/detomidine reversed with atipamezole |
| Telazol (tiletamine/zolazepam) | 1-6 mg/kg IM | Up to 45-60 minutes | Light anesthetic plane Minimal analgesia Useful for non-invasive procedures Zolazepam reversed with flumazenil |
| Telazol + dexmedetomidine OR Telazol + xylazine OR Telazol + detomidine |
1-6 mg/kg Telazol + 0.01-0.02 mg/kg dexmedetomidine IM OR 1-6 mg/kg Telazol + 1-2 mg/kg xylazine IM OR 1-6 mg/kg Telazol + 0.05-0.1 mg/kg detomidine IM |
Up to 60-120 minutes | Light to moderate anesthetic plane Cardiorespiratory depression; oxygen supplementation is recommended Dexmedetomidine/xylazine/detomidine reversed with atipamezole Zolazepam reversed with flumazenil |
| Others | |||
| Detomidine + midazolam + butorphanol (see butorphanol as an analgesic below) |
0.05-0.1 mg/kg detomidine + 0.1-0.2 mg/kg midazolam + 0.2 mg/kg butorphanol IM, IV |
Up to 60 minutes | Profound sedation to light anesthetic plane Useful for non-invasive procedures Midazolam reversed with flumazenil Detomidine reversed with atipamezole Butorphanol reversed with naloxone (if procedure is non-painful) Oxygen supplementation is recommended |
| Propofol | 1-4 mg/kg IV slow bolus 0.2-0.8 mg/kg/min IV constant rate infusion (CRI) |
5-7 minutes | Titrate to effect for anesthetic induction or used as a CRI as part of partial intravenous anesthesia (PIVA) Cardiorespiratory depression; oxygen supplementation is recommended Apnea is common |
*Intramuscular (IM), intravenous (IV). These can be used with opioids to produce analgesia with profound sedation or general anesthesia as below.
Reversal Agents Used in Swine
| Drug | Dosage/Route* | Reversal Category | Reversal For: |
| Atipamezole | 0.05-0.3 mg/kg SC, IM | Alpha-2 adrenergic receptors | Dexmedetomidine Xylazine Detomidine |
| Flumazenil | 0.02-0.08 mg/kg SC, IM, IV | Benzodiazepines | Midazolam Zolazepam |
| Naloxone | 0.1-1 mg/kg SC, IM, IV | Opioids | Buprenorphine, morphine, etc. |
*Subcutaneous (SC), intramuscular (IM), intravenous (IV)
Analgesics
- Examples of common analgesic agents are listed below. Others are available; please contact RARC veterinarians to discuss alternatives such as gabapentin, tramadol, etc.
- Unrelieved pain has profound physiologic consequences, which may alter research results.
- Pain assessment in swine consists of evaluating behavioral and physiologic parameters and use of validated pain scoring systems such as the Unesp-Botucatu Pig Composite Acute Pain Scale (UPAPS) or the Unesp-Botucatu Piglet Composite Acute Pain Scale (UPAPS) (Luna et al 2020; Luna et al 2023).
- Pain scales include abnormal gait and posture, interactions with surroundings, reluctance to move, activity, social isolation, attention to the painful area, tail wagging, pen biting, and appetite. The pain score and other signs should be documented in the record along with any analgesic intervention taken.
- The IACUC requires the use of preemptive analgesia (analgesics given prior to the first skin incision) for all survival surgical procedures unless scientifically justified.
- Pre-emptive analgesics may decrease the amount of required anesthetic drugs; doses of both injectable and inhalant agents should be adjusted accordingly.
Analgesic Agents Used in Swine
| Drug | Dosage/Route* | Frequency | Comments |
| Opioids/Sodium Channel Blockers/NMDA Antagonists | |||
| Buprenorphine (300 mcg/mL) or high concentration buprenorphine (Simbadol™; 1.8 mg/mL) | 0.02-0.05 mg/kg IM, IV | 6-12 hours | Partial mu-opioid agonist Moderate analgesia |
| Compounded extended-release buprenorphine (Buprenorphine-ER) | 0.12-0.24 mg/kg SC | Up to 72 hours | Compounded Associated with skin nodules Partial mu-opioid agonist |
| Morphine | 0.5-1mg/kg IM, slow IV | 4-8 hours | Full mu-opioid agonist Profound analgesia |
| Butorphanol | 0.2-0.5 mg/kg IM, IV | 1-2 hours | Mu-opioid antagonist; kappa-opioid agonist Mild, short analgesia |
| Fentanyl | 2-5 mcg/kg bolus IV 2-20 mcg/kg/hr IV OR 1-2 mcg/kg/hr TD |
CRI OR Patch |
Full mu-opioid agonist Profound analgesia Patch takes up to 18 hr for full effect and can be variable; up to 72 hr duration |
| Lidocaine | 0.5-2 mg/kg bolus IV 50-75 mcg/kg/min IV |
CRI | Sodium channel blockade Anti-inflammatory, reduces amount of inhalant required |
| Ketamine | 0.25-1 mg/kg bolus SC, IV 2-20 mcg/kg/min IV |
CRI | Excellent somatic analgesia Reduces amount of inhalant required |
| NSAIDs and Antipyretics | |||
| Carprofen | 2-4 mg/kg SC, PO | 24 hours | COX-2 selective |
| Meloxicam | 0.4 mg/kg SC, PO | 24 hours | COX-2 selective |
| Phenylbutazone | 5-8 mg/kg PO | 12 hours | Non-selective |
| Flunixin Meglumine | 2.2 mg/kg IV, IM | 24 hours | Non-selective |
| Acetaminophen | 10-30 mg/kg | 24 hours | NOT classified as an NSAID Centrally-acting analgesic May work at COX-3 pathway Antipyretic |
| Local Anesthetics** Doses must be calculated carefully due to toxicity potential with overdose (cardiac, CNS, etc.) |
|||
| Liposomal encapsulated bupivacaine (Nocita®)** | Up to 0.4 mL/kg intra-incisional infiltration | Up to 3 days | Must cover entire incision due to limited diffusion Favorable safety profile |
| Lidocaine** | Up to 6 mg/kg SC | 1-2 hours | Usually not recommended due to short duration of action |
| Bupivacaine** | Up to 2 mg/kg SC | 6-8 hours | Peak onset ~10 minutes |
*Subcutaneous (SC), intraperitoneal (IP), intravenous (IV), oral (PO), transdermal (TD).
**Note: lidocaine and bupivacaine/Nocita® should not be mixed together.
Inhalant Anesthetics
Inhalant anesthetic delivery methods:
- Mask induction is not recommended due to personnel exposure, slow induction times and inability to control the airway. If deemed necessary, sedation should precede mask induction and the diaphragm within the mask must result in a tight seal around the snout.
- Agent-specific vaporizers are required, and excess gas must be scavenged.
- Inhalant should be turned off before mask is removed.
- Anesthetic maintenance using a mask can be performed but is not recommended due to personnel exposure, inability to protect the airway from regurgitation, and inability to control ventilation.
- Endotracheal intubation is recommended during anesthetic maintenance; RARC personnel should be contacted for more information concerning endotracheal intubation in swine.
- A carrier gas is required; usually it is ~100% oxygen.
- Common settings:
- Immediately following induction: ~2 L/min; maintenance: ~1 L/min.
- Common settings:
- Isoflurane and sevoflurane can both be used in swine. However, they require separate vaporizers due to different vapor pressures and the MAC values (see Overview of Anesthetic and Analgesia Procedures tab).
- Common vaporizer settings:
- Isoflurane: immediately following induction ~2-4%, maintenance ~1-2%
- Sevoflurane: immediately following induction ~4-6%, maintenance ~3-4%
- Common vaporizer settings:
- Inhalants are profound cardiovascular and respiratory depressants; premedication is required to smooth induction/recovery and reduce amount of inhalant required.
- Inhalants require proper circle/rebreathing and scavenging systems; RARC personnel can assist in choosing appropriate systems for your laboratory.
- Inhalant anesthetics can trigger Malignant Hyperthermia in genetically susceptible swine. End-tidal CO2 levels and body temperature should be monitored.
Endotracheal Intubation
- See Overview of Anesthetic and Analgesia Procedures tab for additional information. Please consult with RARC personnel if you are performing tracheal intubation in pigs.
- Orotracheal intubation can be difficult because the mouth does not open widely, the oral cavity is small, the view of the larynx is easily obstructed with soft tissue, the tongue is thick, and pharyngeal diverticula are present.
- Adult pig endotracheal tubes are usually 6.0-9.0 mm; piglets require much smaller tubes and depending on age/size can be a small as 4.0 mm.
- Laryngoscopes are used to ensure proper visualization of the larynx for atraumatic intubation. Long fiberoptic laryngoscope blades should be used.
- Pigs should be placed in ventral recumbency with the neck in a natural position. The laryngoscope blade should be placed on the base of the tongue – not on the epiglottis – to visualize the trachea. If the rima glottidis cannot be seen, the soft palate should be displaced off of the epiglottis using a non-metal stylet or the laryngoscope. 2% lidocaine (~ 1 mL in an adult) is placed on the arytenoid cartilages. The endotracheal tube cuff should be lubricated with a sterile, water-soluble lubricant to form an appropriate seal. An introducer may be used; these are non-metal stylets or bougies with blunt ends that are inserted inside the trachea over which the endotracheal tube is passed. If an introducer is not used, the endotracheal tube is placed curved side down, twisted 180° once through the glottis, then twisted another 180° to pass into the trachea due to laryngeal/tracheal diverticula.
- Gentle techniques must be used; trauma is common and laryngeal/tracheal rupture can occur.
- Please contact RARC if you have questions concerning this procedure.
Anesthetic Monitoring
Anesthetic monitoring techniques:
- Although constant vigilance is required during anesthesia (pigs should never be unattended), recording vital signs every 5-10 minutes is recommended to immediately see values and to assess changes over time. RARC can provide you with an anesthetic record template if needed.
- Vital signs
- Cardiovascular system vital signs:
- Heart rate/pulse rate
- Can be taken using a pulse oximeter (see below), palpation of the heart, or stethoscope.
- Normally heart/pulse rates typically are ~70-150 beats/minute, depending on anesthetic agents used.
- Arterial blood pressures
- Measured using oscillometric devices or by placement of direct lines into an artery.
- Mean arterial pressure should always be kept above ~ 70 mmHg to ensure organ perfusion.
- Heart rate/pulse rate
- Respiratory system vital signs:
- Respiratory rate
- This can be measured by visualizing the movement of the animal’s chest wall as it rises and falls or via the end-tidal carbon dioxide monitor (capnometer).
- Normal respiratory rates typically are ~20-40 breaths/minute.
- Capnography
- Veterinary-specific monitors measure end-tidal CO2 levels and produce capnograph waves. These are useful in detecting abnormalities in ventilation and appropriate tracheal intubation.
- This is an essential monitor to detect Malignant Hyperthermia.
- Pulse oximetry
- Veterinary-specific monitors are available to measure the amount of oxygen saturation of hemoglobin in blood (normal = >95%) and pulse rate.
- Readings could be impaired by reduced pulsatile strength (hypotension, hypothermia, vasoconstriction, etc.).
- Respiratory rate
- Anesthetic depth
- Jaw tone, palpebral reflexes, and limb withdrawal reflexes are performed to determine appropriate anesthetic depth prior to a procedure.
- Body temperature
- Pigs can quickly become hypothermic or hyperthermic during anesthesia (normal = 101.5-103.5 °F).
- Rectal or esophageal thermometers or implanted telemeters should be used.
- Temperature should not decrease lower than ~ 98°F throughout the procedure; circulating warm water blankets, forced warm air circulators, and warmed anesthetic circuits should be used.
- Surgical clipping and scrubbing or prepping agents should be minimized.
- Electrical blankets, rice bags, water bottles, and gloves are NOT allowed because they frequently produce burns.
- Pain scoring
- Validated pain scoring systems such as the Unesp-Botucatu Pig Composite Acute Pain Scale (UPAPS) or the Unesp-Botucatu Piglet Composite Acute Pain Scale (UPAPS) should be used and documented in the record.
- Analgesics should be administered as deemed necessary by intervention levels and per protocol or in discussion with RARC veterinary personnel.
- Cardiovascular system vital signs:
Fluid Therapy
- Intra-operative anesthetic fluid rate should be ~3-5 mL/kg/hr IV using balanced crystalloid solutions (Plasmalyte, Normosol, Lactated Ringer’s, etc.). Fluid rates and types should be adjusted based on individual animal needs.
- Voluntary fluid intake should be documented after the procedure into the recovery period.
- Once fully recovered, supplemental fluid can be given IV. Supplemental fluids should consist of a balanced crystalloid (Plasmalyte, Normosol, Lactated Ringer’s, etc.) and may be supplemented with other substances such as glucose.
Recovery
Factors that contribute to a successful recovery:
- Environment
- Recovery observations should be documented every 5-10 minutes until the pig is fully ambulating; a template can be provided by RARC if required.
- Recovery areas should be warm and quiet with dim, yet sufficient lighting to appropriately observe the pigs.
- If appropriate room temperatures cannot be achieved, the incorporation of supplemental heat sources should be used as above.
- A recovery pen with proper padding on the walls and floor (when they are in recumbency) is required. An impermeable surface or rubber mat will help as they regain footing and ambulation.
- Pigs should be extubated at the return of the swallow reflex and when they can maintain sternal recumbency.
- Proper hydration and GI function
- Supplemental fluid support (as described above), nutritional support (moist food, enriched food, etc.), and/or active feeding techniques may be necessary and beneficial. Palatable food should be offered when pigs are ambulating and have fully regained their swallow reflex to reduce GI stasis.
- Like fluid intake, food intake should be documented in the record and monitored regularly.
Swine Validated Pain Scoring System
Pain Scale: Unesp-Botucatu Pig Pain Scale
|
ITEM |
Description |
Score |
|
Posture: |
||
|
1 |
Normal (any position, apparent comfort, relaxed muscles) or sleeping |
0 |
|
Changes posture, with discomfort |
1 |
|
|
Changes posture, with discomfort, and protects the affected area |
2 |
|
|
Quiet, tense, and back arched |
3 |
|
|
Interaction and interest in the surroundings: |
||
|
2 |
Interacts with other animals; interested in the surroundings or sleeping |
0 |
|
Only interacts if stimulated by other animals; interested in the surroundings |
1 |
|
|
Occasionally moves away from other animals, but accepts approaches; shows little interest in surroundings |
2 |
|
|
Moves or runs away from other animals and does not allow approaches; disinterested in the surroundings |
3 |
|
|
Activity: |
||
|
3 |
Moves normally or sleeping |
0 |
|
Moves with less frequency |
1 |
|
|
Moves constantly; restless |
2 |
|
|
Reluctant to move or does not move at all |
3 |
|
|
Appetite: |
||
|
4 |
Normal appetite (normorexia) |
0 |
|
Increased appetite (hyperexia) |
1 |
|
|
Decreased appetite (hyporexia) |
2 |
|
|
No appetite (anorexia) |
3 |
|
|
Attention to the affected area (check which of the following apply): |
||
|
5 |
A) Elevates the pelvic limb or alternates the support of the pelvic limb |
|
|
B) Scratches or rubs the painful area |
||
|
C) Moves and/or runs away and/or jumps after injury of the affected area |
||
|
D) Sits with difficulty |
||
|
SCORING: All of the above behaviors described in A, B, C, and D are absent |
0 |
|
|
Presence of one of the behaviors described in A, B, C, or D |
1 |
|
|
Presence of two of the behaviors described in A, B, C, and D |
2 |
|
|
Presence of three or all of the behaviors described in A, B, C, and D |
3 |
|
|
Miscellaneous Behaviors (check which of the following apply): |
||
|
5 |
A) Wags tail continuously and intensely |
|
|
B) Bites the bars or objects |
||
|
C) The head is below the line of the spinal column |
||
|
D) Presents difficulty in overcoming obstacles |
||
|
SCORING: All of the above behaviors described in A, B, C, and D are absent |
0 |
|
|
Presence of one of the behaviors described in A, B, C, or D |
1 |
|
|
Presence of two of the behaviors described in A, B, C, and D |
2 |
|
|
Presence of three or all of the behaviors described in A, B, C, and D |
3 |
|
Scale and Decision for Analgesic:
- Score total can range from 0 to 18
- Analgesic intervention given at a score ≥ 4
Additional Resources
Consult the RARC Veterinary Staff (vet@rarc.wisc.edu) if you have specific questions about the anesthetic procedures included in your IACUC protocol.
For assistance with anesthesia equipment set-up, please contact the RARC Trainers (trainer@rarc.wisc.edu).
Form templates to record anesthesia monitoring are available on the RARC website.
RARC loans out select anesthesia equipment to the UW-Madison research community for temporary use. Please visit the RARC website to find out what items are available to borrow.
Useful Text Resources:
Veterinary Anesthetic and Monitoring Equipment by Cooley and Johnson
UW-Madison Libraries provides free access to the online version of this textbook.
Veterinary Anesthesia and Analgesia: The Sixth Edition of Lumb and Jones by Lamont et al.
UW-Madison Libraries provides free access to the online version of this textbook.
Exotic Animal Formulary by Carpenter et al.
UW-Madison Libraries provides free access to the online version of this textbook.
Works Cited
“Anesthesia (Guideline).” Anesthesia (Guideline)|Vertebrate Animal Research, 10 May 2023. https://animal.research.uiowa.edu/iacuc-guidelines-anesthesia.
Danneman PJ and Fish RE. Anesthesia and Analgesia in Laboratory Animals (Second Edition). Academic Press, 2008.
Flecknell PA. Laboratory Animal Anaesthesia Ed. 4. Academic Press, 2015.
Lamont L et al. Veterinary Anesthesia and Analgesia: The Sixth Edition of Lumb and Jones. Wiley Blackwell, 2024.
Luna SPL, de Araújo AL, da Nóbrega Neto PI, Brondani JT, de Oliveira FA, Azeredo LMDS, Telles FG, Trindade PHE. 2020. Validation of the UNESP-Botucatu pig composite acute pain scale (UPAPS). PLoS One. Jun 1;15(6):e0233552. doi: 10.1371/journal.pone.0233552. eCollection 2020.
Robles I, Luna SPL, Trindade PHE, Lopez-Soriano M, Merenda VR, Viscardi AV, Tamminga E, Lou ME, Pairis-Garcia MD. 2023. Validation of the Unesp-Botucatu pig composite acute pain scale (UPAPS) in piglets undergoing castration. PLoS One. Apr 13;18(4):e0284218. doi: 10.1371/journal.pone.0284218. eCollection 2023.
“Supplemental Anesthesia Training Resource.” Received by Rebecca Johnson, Supplemental Anesthesia Training Resource, 7 May 2020.